A kind of recombinant Escherichia coli with high production of l-threonine and its construction method
A technology for recombining Escherichia coli and Escherichia coli, which is applied in the field of genetic engineering, can solve problems such as doubts about the use of antibiotics, and achieve the effects of reducing metabolic consumption, ease of use, and simple construction methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Construction of embodiment 1 recombinant fragment
[0080] According to the Escherichia coli sequence information, design primers pT-thrB-1R and pT-thrB-2F (see Table 3), according to the Escherichia coli CICC20905 genome sequence information, design primers pT-thrB-1F, pT-thrB containing T7 promoter sequence -2R, using the above four primers to amplify 600 bp of the thrABC homology arm gene sequence with the T7 promoter from the genome of Escherichia coli CICC20905, and fuse the two amplified fragments obtained by fusion PCR technology to obtain the recombinant fragment T71;
[0081] According to the sequence information of Escherichia coli, primers pT-tdc-1F, pT-tdc-1R, pT-tdc-2F and pT-tdc-2R were designed, and the homology arms on both sides of the tdcB gene were amplified from the genome of Escherichia coli CICC20905 using the above primers The gene sequence is 600bp each, and the obtained two amplified fragments are fused by fusion PCR technology to obtain the rec...
Embodiment 2
[0086] The construction of embodiment 2 recombinant plasmids
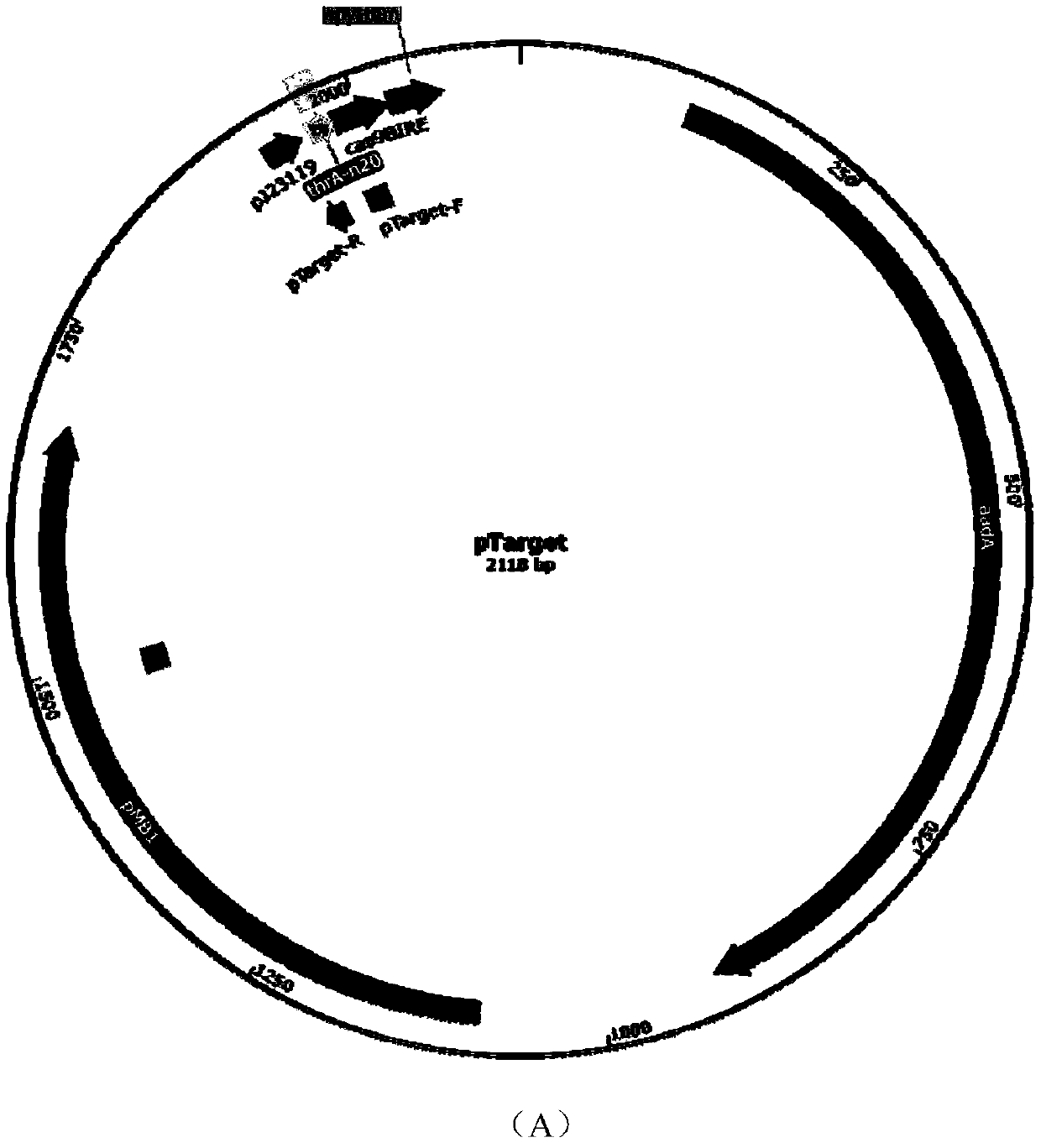
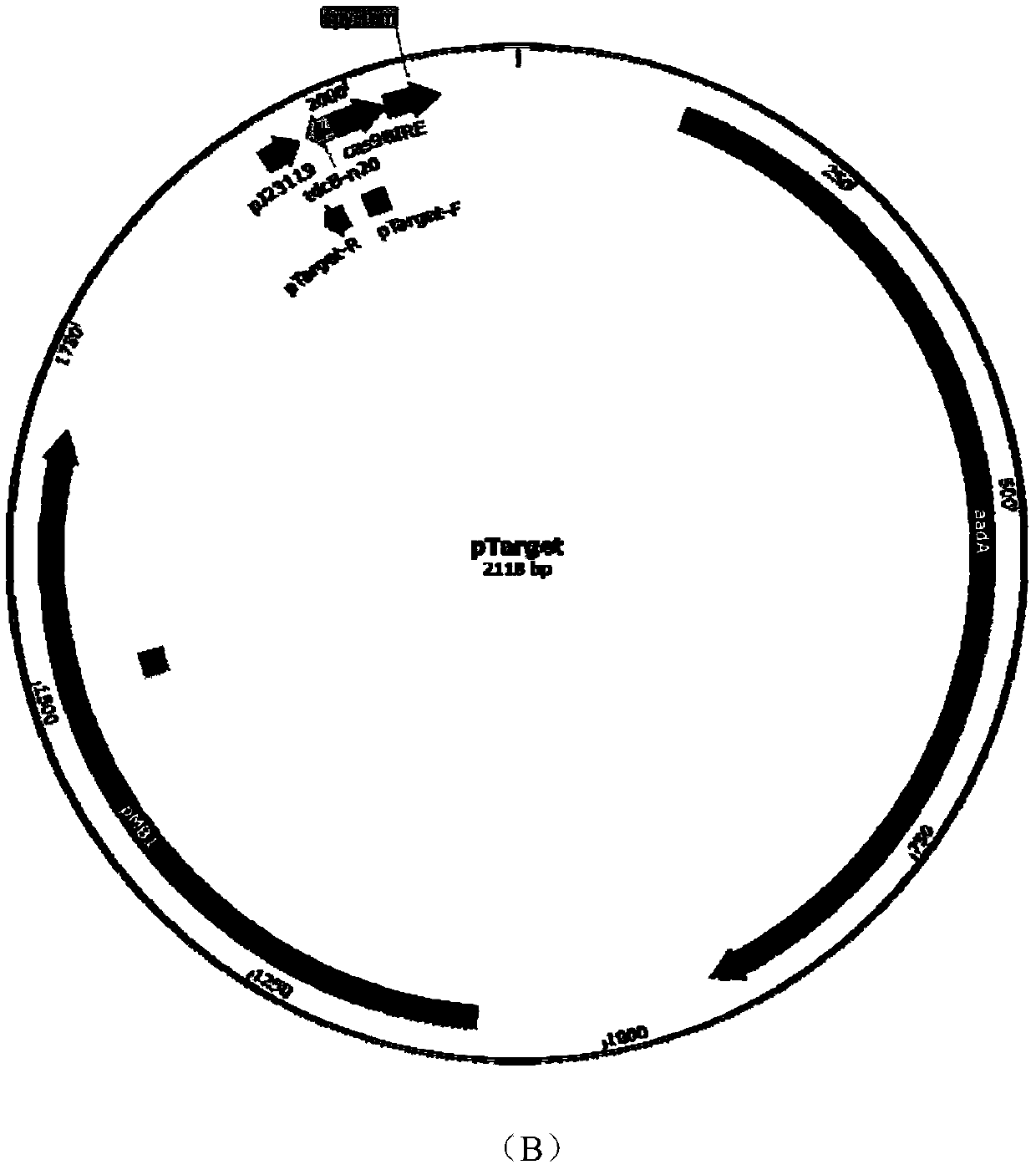
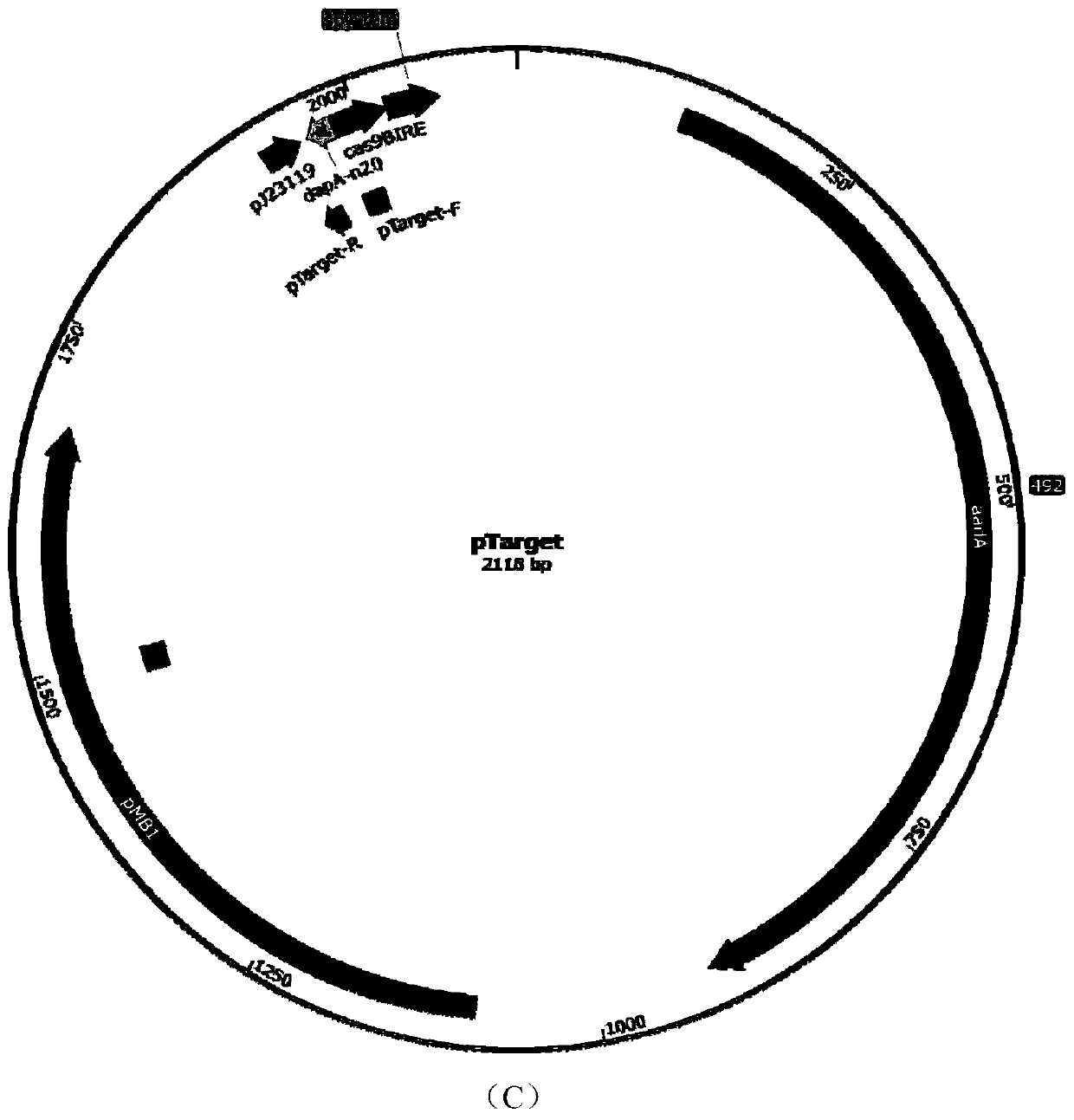
[0087] According to the vector pTarget sequence information, design primers pT-thrB-F, pT-thrB-R and carry out PCR to obtain the linearized vector pTT7 containing sgRNA (sequence information is shown in SEQ ID NO.5); design primers pT-tdc-F and pT-tdc-R was carried out PCR to obtain the linearized carrier pTTD (sequence information as shown in SEQ ID NO.6) containing sgRNA; Design primers pTGTG-F and pT-GTG-R to carry out PCR to obtain the linearized carrier pTDA containing sgRNA ( The sequence information is shown in SEQ ID NO.7), respectively connected with the recombinant fragments T71, TD2, and DA3 to construct recombinant plasmids pTT7, pTTD, pTDA, BamHI, BsgI double enzyme digestion verification and sequencing, confirming that the recombinant plasmids were successfully constructed. The plasmid maps of pTT7, pTTD, pTDA are as follows figure 1 shown.
Embodiment 3
[0088] Example 3 Construction of Recombinant T71 Fragment Escherichia coli
[0089] The pCas9 plasmid containing cas9 protein was transformed into Escherichia coli CICC20905. The Kana resistance plate was used to screen the successfully transformed recombinant Escherichia coli CGC9, and then the recombinant plasmid pTT7 was transformed into Escherichia coli CICC20905-cas9, the screening confirmed the successful integration of the T7 promoter, and 0.05mM IPTG was added to induce at 30°C for 12 hours to remove the recombinant plasmid pTT7, The primers pT-thrB-2F and pT-thrB-2R were used to select the transformants for colony PCR, and a band of about 600 bp appeared. After the sequence was correct, the recombinant E. coli CICC20905-thrT with the thrABC gene cluster promoter T7 was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com