Aromatic ring ureidindole derivatives and their preparation methods and applications
A technology of aromatic ring ureidindole derivatives and alkyl groups, which is applied in the field of aromatic ring ureidindole derivatives and their preparation, can solve the problems of low overall yield, poor stability, and long steps in the synthesis route, and achieve improved Sensitivity, reversal of drug resistance, and simple synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] The present invention also provides a preparation method of aromatic ring ureidindole derivatives, comprising:
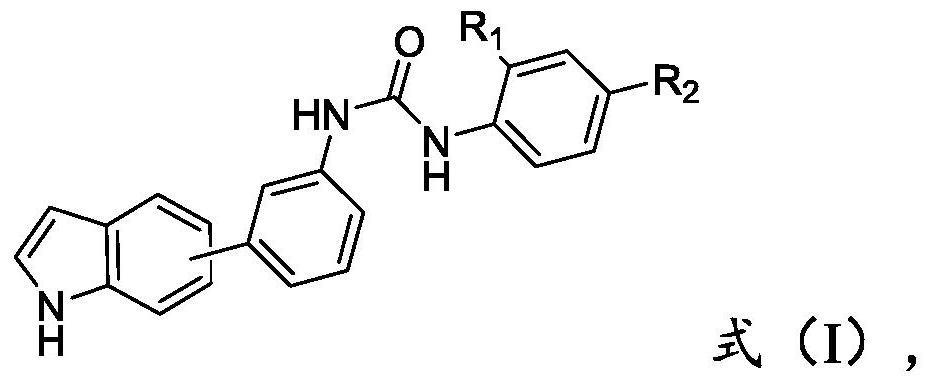
[0020] The compound of formula (II) structure and the compound of formula (III) structure are reacted, obtain the compound of formula (I) structure,
[0021]
[0022] where the R 1 , R 2 are independently selected from hydrogen, C1-C8 alkyl or C1-C8 alkoxy.
[0023] According to the present invention, the present invention reacts the compound of formula (II) structure and the compound of formula (III) structure, obtains the compound of formula (I) structure, wherein, the compound of described formula (II) structure and described formula (III) The molar ratio of the compound of ) structure is preferably 1: (0.9~1.1), more preferably 1: 1; The auxiliary agent of described reaction is preferably triethylamine; The solvent of described reaction is preferably ethanol, methanol and propanol One or more of them; the reaction is preferably carried out at room t...
Embodiment 1
[0033] Synthesis of Intermediate Compounds II-3, II-4
[0034] In a 25 mL double necked round bottom flask, add 2 mL of Na 2 CO 3 solution (1mol / L), 5mL of Toluene-C 2 h 5 OH (v / v, 1:1), 1.0mmol of IV-1 or IV-2, 2.0mmol of 3-aminophenylboronic acid-hydrate, 0.05mmol of Pd(PPh 3 ) 4 , remove the O in the system by vacuuming 2 , N 2Reflux reaction at 80°C under protection until the reaction of raw materials is complete. The reaction solution was cooled, filtered with suction, and the filtrate was collected, dried, and column chromatographed to obtain compound II-3 (yield 78.3%) or II-4 (yield 72.9%).
Embodiment 2
[0036] The synthetic method of target compound I-a~I-d
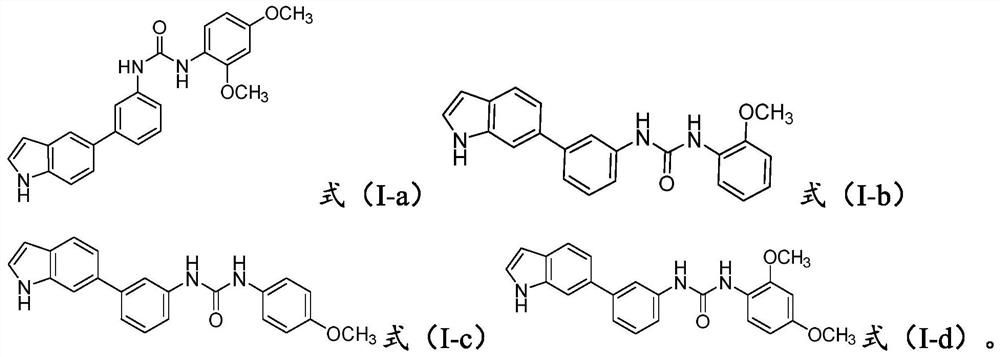
[0037] Synthesis of Compound I-a
[0038] In a 25 mL double necked round bottom flask, add 1.0 mmol of II-3, 1.0 mmol of III-c, 3.0 mmol of Et 3 N, 10 mL of C 2 h 5 OH, react at room temperature, and stop the reaction until the conversion of raw materials is complete. The reaction solution was concentrated and dried, and 224 mg of the product was obtained by column chromatography, with a yield of 57.8%.
[0039] Compound I-a provided by the invention is a white solid, molecular formula C 23 h 21 N 3 o 3 , molecular weight 387.15829. Chinese name 1-(3-(1H-indol-5-yl)phenyl)-3-(2,4-dimethoxyphenyl)urea, English: 1-(3-(1H-indol-5 -yl)phenyl)-3-(2,4-dimethoxyphenyl)urea; 1 H NMR (400MHz, Acetone-d 6 )δ10.26(s, 1H), 8.58(s, 1H), 8.12(d, J=8.9Hz, 1H), 7.89(t, J=1.9Hz, 1H), 7.81(dd, J=1.8, 0.9 Hz, 1H), 7.67(s, 1H), 7.50-7.36(m, 3H), 7.34-7.21(m, 3H), 6.58-6.43(m, 3H), 3.82(s, 3H), 3.73(s, 3H); 13 C NMR (100MHz, Ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com