Methods for treating inflammatory conditions of the lungs
An inflammatory disease, lung technology, applied in chemical instruments and methods, anti-inflammatory agents, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
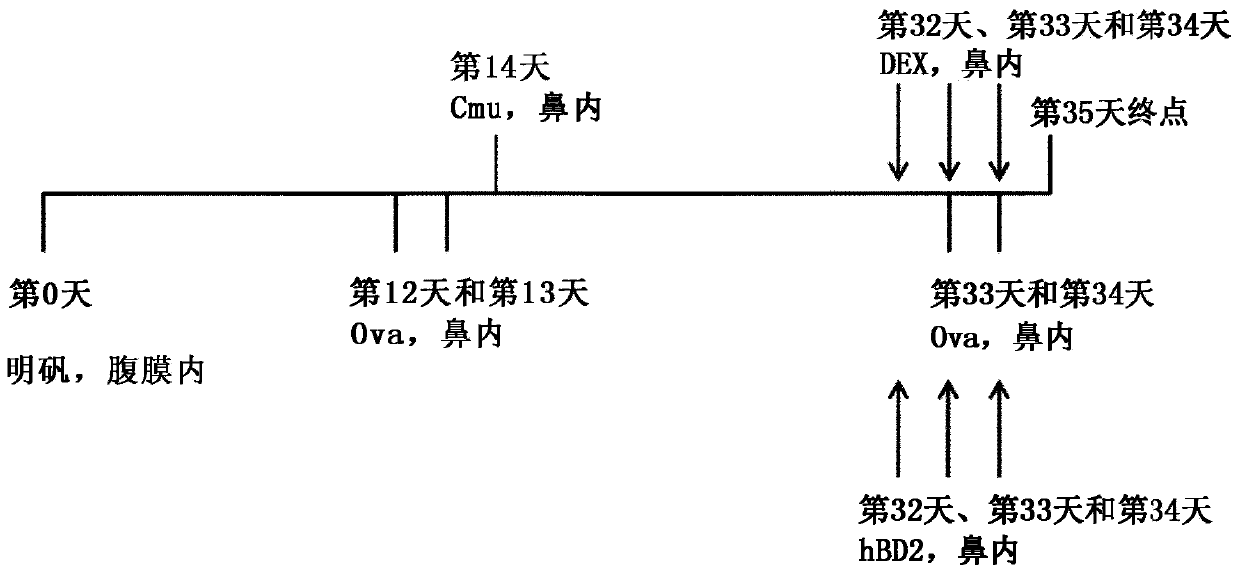
[0222] To identify and evaluate the efficacy of mammalian β-defensins in a murine infection-induced asthma model of severe steroid insensitivity, neutrophilic, allergic airway disease ( figure 1 ).
[0223] Materials and methods
[0224] treatment plan :
[0225]Female 6-8 week old BALB / c mice were sensitized intraperitoneally (IP) with 50 μg ovalbumin in 200 μL 0.9% saline with 1 mg adjuvant alum. Mice were challenged intranasally (IN) with Ova (10 μg Ova in 50 μL sterile saline) on days 12-13 and 33-34. On day 14, mice were inoculated intranasally with the natural mouse pathogen Chlamydia muridarum (Cmu: 100 inclusion body forming units, ATCC VR-123, 30 μL sucrose phosphate glutamate buffer (SPG)). Dexamethasone (DEX) (2 mg / kg; 50 μL phosphate-buffered saline (PBS)) was administered intranasally on days 32-34 of the challenge with Ova. hBD-2 (5 mg / kg; 50 μL phosphate-buffered saline) was administered intranasally on days 30, 32 and 34.
[0226] Drugs administered to m...
Embodiment 2
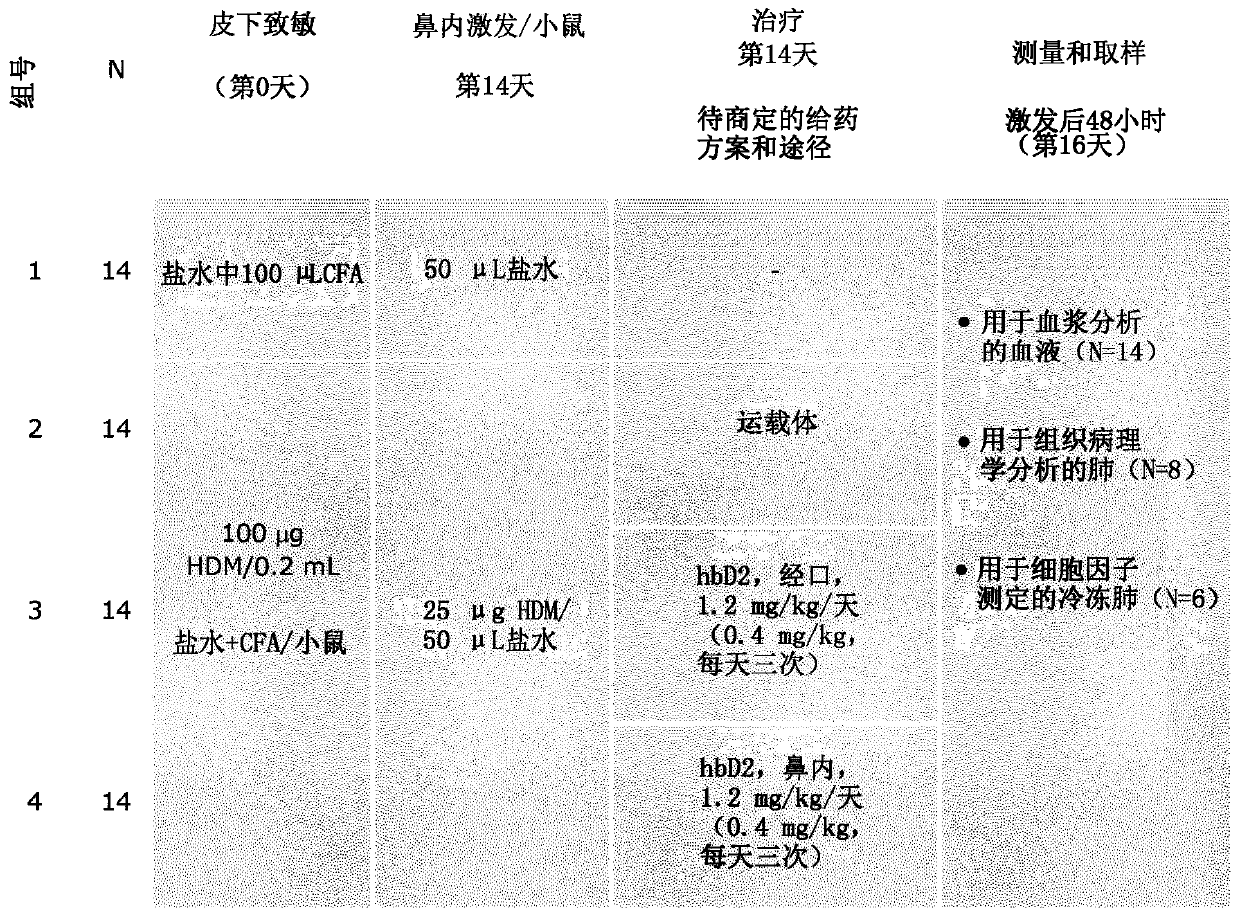
[0235] To determine and evaluate the efficacy of intranasally and orally administered mammalian β-defensins in a murine house dust mite / Freund's complete adjuvant-driven model of allergic asthma ( figure 2 ).
[0236] Materials and methods
[0237] treat Protocol: Female 7-10 week-old BALB / c mice were randomly assigned to 7 study groups one day before the start of the study and were infested with house dust mites (100 μg HDM in 200 μL saline plus Freund’s in 0.9% saline complete adjuvant) subcutaneous (SC) sensitization. Mice were then challenged intranasally (IN) on day 14 with HDM (25 μg HDM in 50 μL saline). On day 14, dexamethasone (1 mg / kg twice daily; 50 μL phosphate buffered saline (PBS)) was administered orally. hBD-2 was administered intranasally or orally on day 14 (1.7 mg / kg three times a day, intranasal; 0.4 mg / kg three times a day, intranasal; 0.4 mg / kg three times a day, orally, 50 μL phosphate-buffered saline ). The initial dose was administered 60 minut...
Embodiment 3
[0248] To identify and evaluate the efficacy of intranasal and oral mammalian β-defensins in a murine house dust mite / Freund's complete adjuvant-driven model of allergic asthma ( figure 2 ).
[0249] Materials and methods
[0250] treatment plan : Female 7-10 week-old BALB / c mice randomly assigned to one of the 4 study groups on the day before the start of the study were infected with house dust mites (100 μg HDM in 200 μL saline plus Freund’s complete adjuvant in 0.9% saline ) subcutaneous (SC) sensitization. Mice were challenged intranasally (IN) with HDM (25 μg HDM in 50 μL saline) on day 14. On day 14, hBD-2 was administered intranasally or orally (0.4 mg / kg three times a day intranasal; orally 0.4 mg / kg three times a day in 50 μL phosphate-buffered saline). The initial dose was administered 60 minutes prior to challenge, and subsequent doses were spaced approximately 6 hours apart.
[0251] test:
[0252] Lung Tissue Sampling: Lungs were removed from the chest c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap