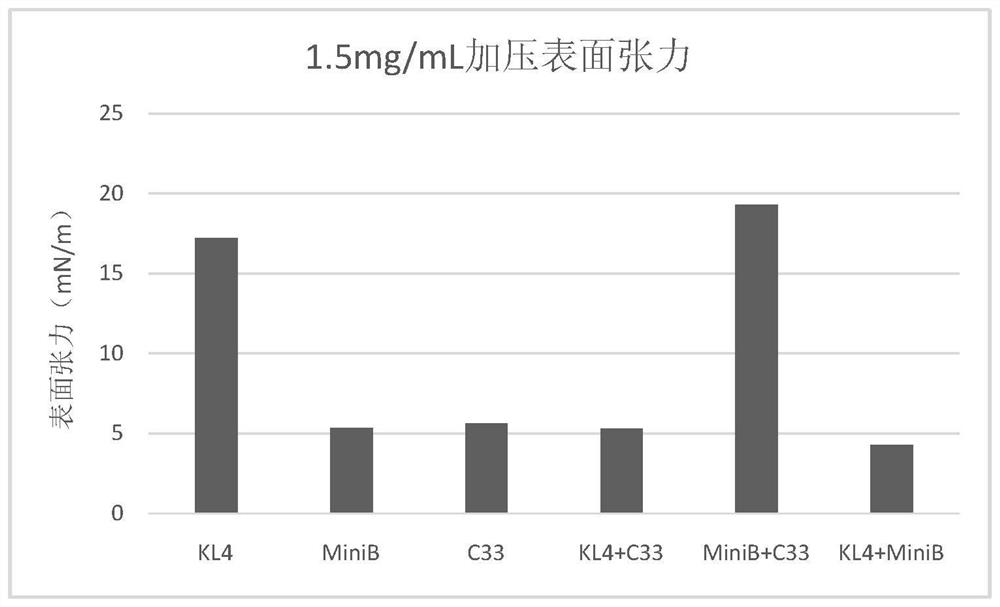
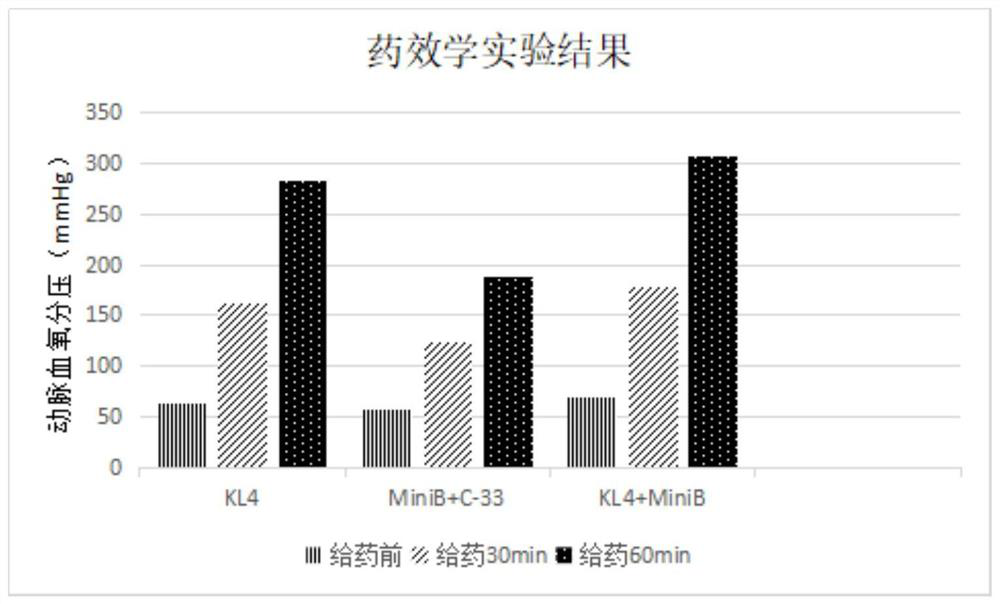
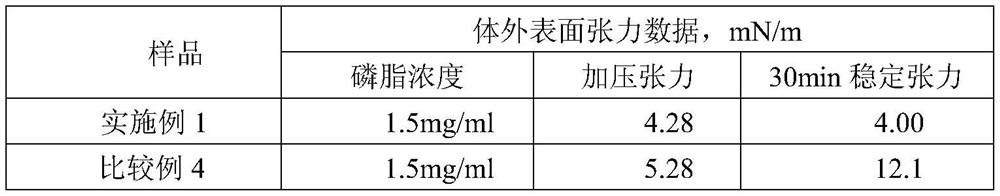
New-generation synthetic pulmonary surfactant preparation and clinical application thereof
A pulmonary surfactant and preparation technology, applied in the field of new-generation synthetic pulmonary surfactant, can solve the problems of poor stability and low activity of fully synthetic PS preparations, achieve stable and long-lasting curative effect, increase in vitro surface activity, and improve blood oxygen level. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] According to the information corresponding to Example 1 in the above table, add all the materials into ethanol (95%, v / v) at 35-45°C, stir until dissolved, and filter while hot; add a buffer solution (tris or Phosphate, sodium chloride, calcium chloride, etc.) (pH = 7.0 ± 0.5), stir quickly until uniform, and remove the organic solvent by circular dialysis or nanofiltration until the solvent residue meets the requirements of the relevant regulations of the Chinese Pharmacopoeia.
[0051] Detect the pH and content of the medicinal solution, supplement the buffer solution according to the test results, and adjust the pH to the specified level (pH=7.0±0.5) if necessary. When necessary, appropriate filtration or other means can be used to remove microorganisms in the liquid medicine.
[0052] Quantitative filling into borosilicate glass control injection bottles (or other pharmaceutical packaging materials) to prepare PS preparations.
Embodiment 2
[0054] According to the information corresponding to Example 2 in the above table, add all the materials into isopropanol (90%, v / v) at 35-45° C., stir until dissolved, and filter while hot; add the buffer solution ( tris or phosphate, sodium chloride, calcium chloride, etc.), stir rapidly until uniform, remove the organic solvent by gradient centrifugation, freeze-dry, and moderately pulverize (or micronize) to obtain the PS preparation.
Embodiment 3
[0056] According to the information corresponding to Example 3 in the above table, all the materials were added into the acidified methanol-chloroform solution kept at 35-45° C., stirred evenly, and filtered.
[0057] After blowing dry with nitrogen gas, oscillate evenly with a buffer solution (tris or phosphate, etc.), and quantitatively dispense into a suspension dosage form. It can also be freeze-dried to produce a freeze-dried product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com