Monocyclic oga inhibitor compounds
A compound and selected technology, applied in organic chemistry, drug combination, nervous system diseases, etc., can solve problems such as cell cycle arrest and glycogen mobilization defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
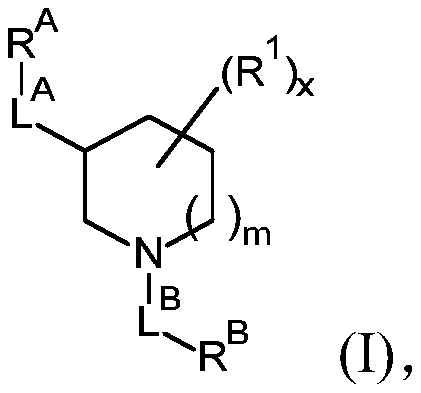
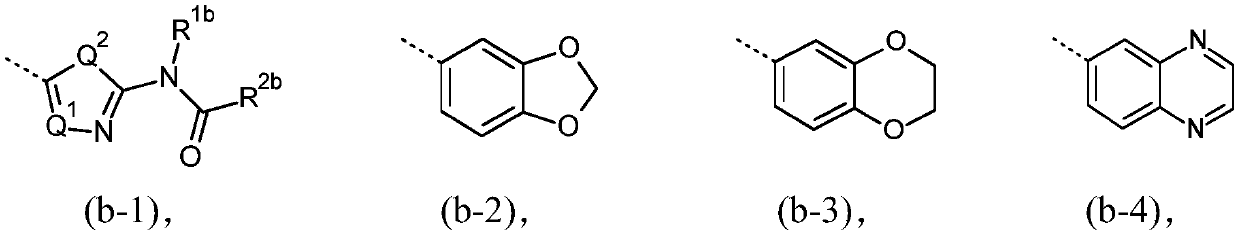
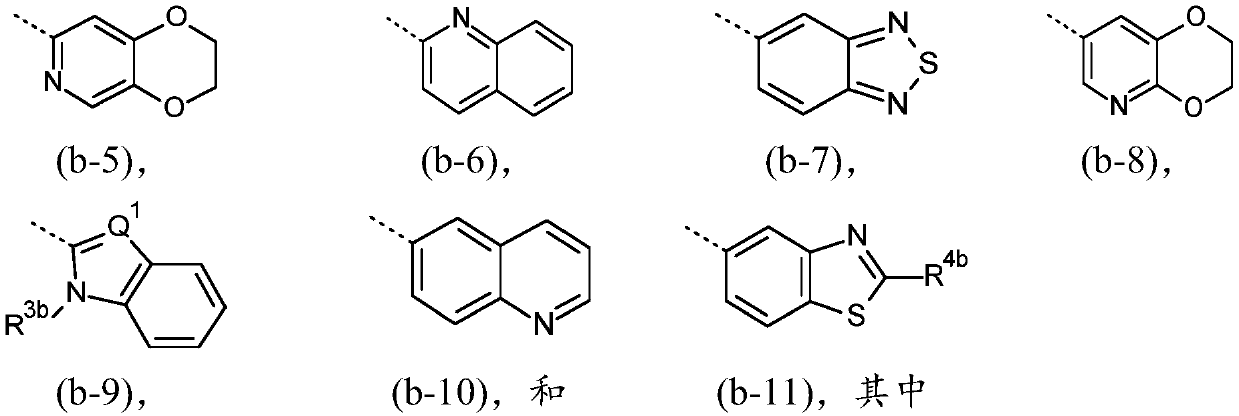
[0114] The present invention relates to a compound of formula (I), or a compound of formula (I'), and pharmaceutically acceptable addition salts and solvates thereof, for use as defined above. Compounds of formula (I) are inhibitors of O-GlcNAc hydrolase (OGA) and are useful for the prophylaxis or treatment of tauopathies, in particular tauopathies selected from the group consisting of: Alzheimer progressive supranuclear palsy, Down syndrome, frontotemporal dementia, frontotemporal dementia with parkinsonism-17, Pick's disease, corticobasal degeneration, and argyrophilic granular disease; or available For the prevention or treatment of neurodegenerative diseases associated with tauopathies, especially neurodegenerative diseases selected from amyotrophic lateral sclerosis or frontotemporal dementia caused by C9ORF72 mutations.
[0115] In a particular embodiment, the invention relates to compounds of formula (I') as defined above, and tautomeric and stereoisomeric forms th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com