Application of composition in preparation of medicine for treating amyotrophic lateral sclerosis
A technology for lateral sclerosis and muscular atrophy, which is applied in the directions of drug combination, active ingredients of hydroxyl compounds, and pharmaceutical formulations, and can solve problems such as inability to judge and predict therapeutic effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
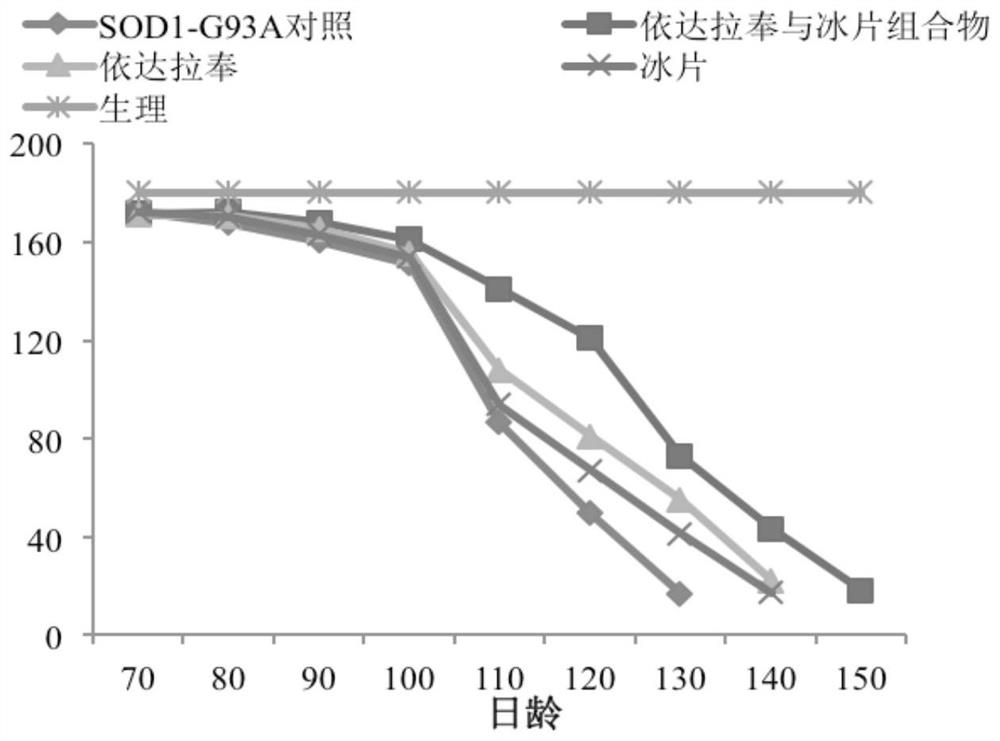
[0032] Example 1: Research on the therapeutic effect of the composition of edaravone and borneol on transgenic amyotrophic lateral sclerosis mice
[0033] 1 Materials and methods
[0034] 1.1 Test animals
[0035] Transgenic mice (B6SJL-TgN[SOD1-G93A]1Gur) carrying human copper / zinc superoxide dismutase (SOD1) (G93A mutant) and wild-type B6SJL F1 mice were purchased from Nanjing University. Breeding and breeding methods of mice: A wild-type female mouse is mated with a transgenic male mouse, and the offspring are identified by polymerase chain reaction (PCR) using the DNA extracted from the tail of the young mouse.
[0036] 1.2 Drugs and reagents
[0037] Edaravone and Borneolum composition, mass ratio 4:1 (described Borneolum is 2-borneol);
[0038] Edaravone (chemical name: 3-methyl-1-phenyl-2-pyrazolin-5-one);
[0039] Borneol (chemical name: 2-borneol).
[0040] 1.3 Test method
[0041] 1.3.1 Breeding and screening of B6SJL-TgN(SOD1-G93A)1Gur mice:
[0042] Five B6S...
Embodiment 2
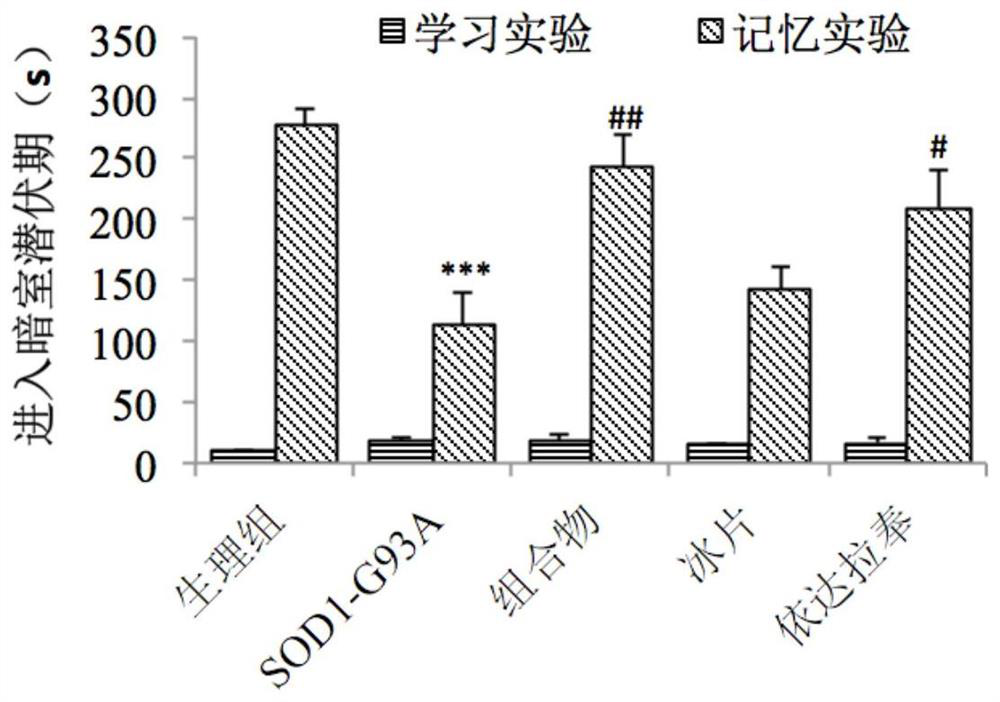
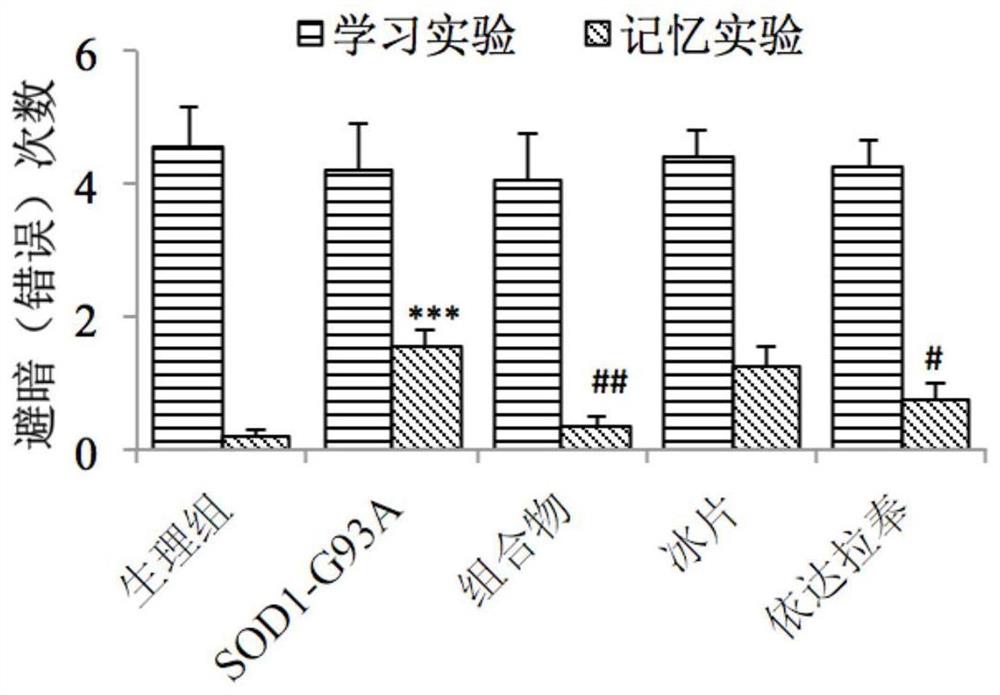
[0064] Example 2: Effect of Edaravone and Borneol Composition on Learning and Memory of Transgenic Amyotrophic Lateral Sclerosis Mice
[0065] 1 Materials and methods
[0066] 1.1 Test animals
[0067] Transgenic mice (B6SJL-TgN[SOD1-G93A]1Gur) carrying human copper / zinc superoxide dismutase (SOD1) (G93A mutant) and wild-type B6SJL F1 mice were purchased from Nanjing University. Breeding and breeding methods of mice: A wild-type female mouse is mated with a transgenic male mouse, and the offspring are identified by polymerase chain reaction (PCR) using the DNA extracted from the tail of the young mouse.
[0068] 1.2 Drugs and reagents
[0069] Edaravone and borneol composition (the borneol is 2-borneol);
[0070] Edaravone (chemical name: 3-methyl-1-phenyl-2-pyrazolin-5-one);
[0071] Borneol (chemical name: 2-borneol).
[0072] 1.3 Test method
[0073] 1.3.1 Breeding and screening of B6SJL-TgN(SOD1-G93A)1Gur mice:
[0074] Five B6SJL-TgN(SOD1-G93A)1Gur male mice were m...
Embodiment 3
[0091] Example 3 Study on Therapeutic Effect of Edaravone and Borneolum Composition on Amyotrophic Lateral Sclerosis Transgenic Mice
[0092] 1 Materials and methods
[0093] 1.1 Test animals
[0094] Amyotrophic lateral sclerosis transgenic mice (B6SJL-Tg(SOD1*G93A)1Gur / JNju, hereinafter referred to as SOD1-G93A mice) and wild-type control mice were purchased from Nanjing University-Nanjing Institute of Biomedicine.
[0095] 1.2 Drugs and reagents
[0096] Edaravone and natural borneol composition (the mass ratio of edaravone and natural borneol is 4:1)
[0097] Edaravone
[0098] Riluzole
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com