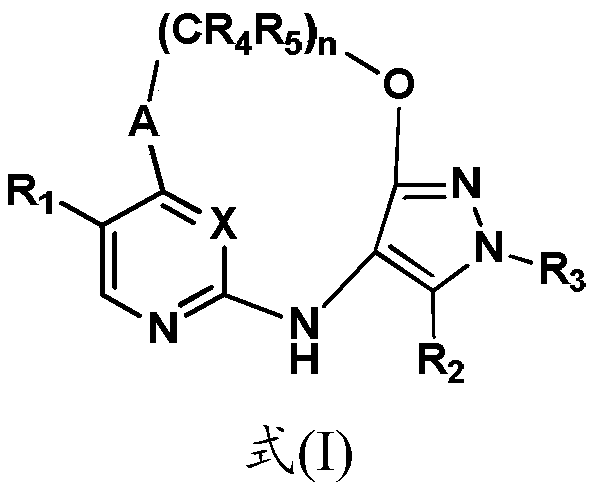
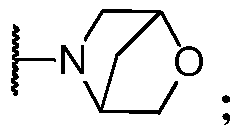
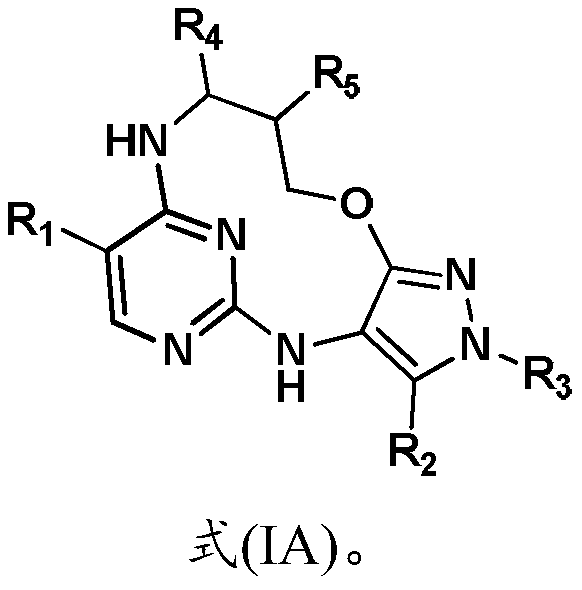
Inhibitors of leucine rich repeat kinase 2
A compound, selected technology, applied in the direction of medical preparations containing active ingredients, drug combinations, organic chemistry, etc., can solve the problems of reduced axon length and branching, defects in cell proliferation and migration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0748] General Experimental Methods
[0749] The following description and examples illustrate the invention. These examples are not intended to limit the scope of the invention, but to provide guidance to those skilled in the art in making and using the compounds, compositions and methods of the invention. While specific embodiments of the invention have been described, it will be understood by chemists in the art that various changes and modifications can be made without departing from the spirit and scope of the invention.
[0750] The chemical names of the compounds described in this application follow the IUPAC nomenclature.
[0751] Heating of the reaction mixture by microwave irradiation was accomplished on a Smith Creator (purchased from Personal Chemistry, Forboro / MA, now owned by Biotage), Emrys Optimizer (commercially available from Personal Chemistry), or Explorer (courtesy of CEM Discover, Matthews / NC) microwaves.
[0752] Conventional techniques can be used h...
Embodiment E1
[1669] 14-Chloro-4-methyl-5-(oxan-4-yl)-8-oxa-2,5,6,12,16,17-hexaazatricyclo[11.3.1.0 3,7 ]Heptadeca-1(16),3,6,13(17),14-pentaene(E1)
[1670]
[1671] To a solution of D9 (121 mg, 0.30 mmol) in i-PrOH (5 mL) was added HCl (0.03 mL, 0.3 mmol). The reaction was stirred at 100°C for 16 hours. The reaction mixture was concentrated and the residue was diluted with EtOAc (20 mL) and washed with saturated NaHCO 3 Wash with aqueous solution (20 mL). The organic layer was then concentrated and the residue was purified by prep-HPLC to give the title compound as a white solid (29 mg, 26.4% yield). LC-MS: 367.5[M+H] + . 1 H NMR (400MHz, CDCl 3 ): δ7.81(s, 1H), 6.26(s, 1H), 5.55(s, 1H), 4.40(br, 2H), 4.12~4.10(m, 3H), 3.48~3.36(m, 4H), 2.29-2.21 (m, 5H), 1.92 (br, 2H), 1.80-1.74 (m, 2H).
Embodiment E2
[1673] 14-Chloro-10-methoxy-4-methyl-5-(oxan-4-yl)-8-oxa-2,5,6,12,16,17-hexaazatri ring [11.3.1.0 3,7 ]Heptadeca-1(16),3,6,13(17),14-pentaene(E2)
[1674]
[1675] To a solution of D16 (120 mg, 0.26 mmol) in EtOH (20 mL) was added Fe (72 mg). Then add NH 4 Cl (68.9mg) in H 2 solution in O (2 mL). The reaction was stirred overnight at 100 °C. The mixture was filtered and the filter cake was washed with EtOH (2 x 50 mL). The combined filtrates were concentrated to give the title compound as a white solid (40 mg, 38.9% yield). LC-MS: 395.3 [M+H] + . 1 H NMR (400MHz, CDCl 3 ): δ7.85(s, 1H), 6.07(s, 1H), 5.68(br, 1H), 4.50(d, J=7.6Hz, 1H), 4.34~4.29(m, 1H), 4.12~4.06( m, 3H), 3.90(d, J=2.0Hz, 1H), 3.52(t, J=11.6Hz, 2H), 3.40(s, 3H), 3.36~3.27(m, 2H), 2.32~2.20(m , 5H), 1.81 ~ 1.75 (m, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com