Enzyme-catalyzed asymmetric synthesis of key intermediates of dextromethorphan
A kind of technology of dextromethorphan hydrobromide and intermediate, which is applied in the field of preparation of key intermediates of antitussives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the acquisition of highly expressed genetically engineered bacteria
[0024] The whole gene synthesis was completed by Shanghai Xuguan Company.
[0025] According to the gene AtIR of Amycolatopsis thermoflava (WP_027931121.1), the gene StIR of Streptomycesthermoilacinus (WP_023587323.1), the gene PmIR of Prauserella marina (WP_091804541.1), the gene SmIR of Sciscionella marina (WP_020496004.1 of Amyalcolatops gene), WP_020635634.1) and the gene ShIR (SHE96216.1) of Streptoalloteichus hindustanus were respectively codon-optimized in order to enable the gene to be expressed in the E. coli expression host. See the attached table for the sequence. And add corresponding enzyme cutting sites at both ends of the gene, and construct them into corresponding vectors to obtain genetically engineered bacteria IR1, IR2, IR3, IR4, IR5, and IR6.
[0026] Transform the prepared recombinant vector into Escherichia coli BL21, Rosetta or Origami by conventional methods to c...
Embodiment 2
[0027] The cultivation of embodiment 2 genetically engineered bacteria and the preparation of resting cells
[0028]Pick a single colony on the plate and inoculate it into 5ml of fermentation medium containing corresponding antibiotics, cultivate it for about 15 hours as a seed solution, inoculate it into 600ml of fermentation medium according to the inoculation amount of 1%, and cultivate it on a shaker at 37°C and 200rpm to OD 600 = about 0.6-0.8, add IPTG with a final concentration of 0.1 mM to induce for more than 10 h, and collect the bacteria by centrifuging the culture solution at 8000 rpm.
Embodiment 3
[0029] Embodiment 3 Utilizes the asymmetric reduction of resting cells of IR1 to catalyze formula (1) hydrochloride
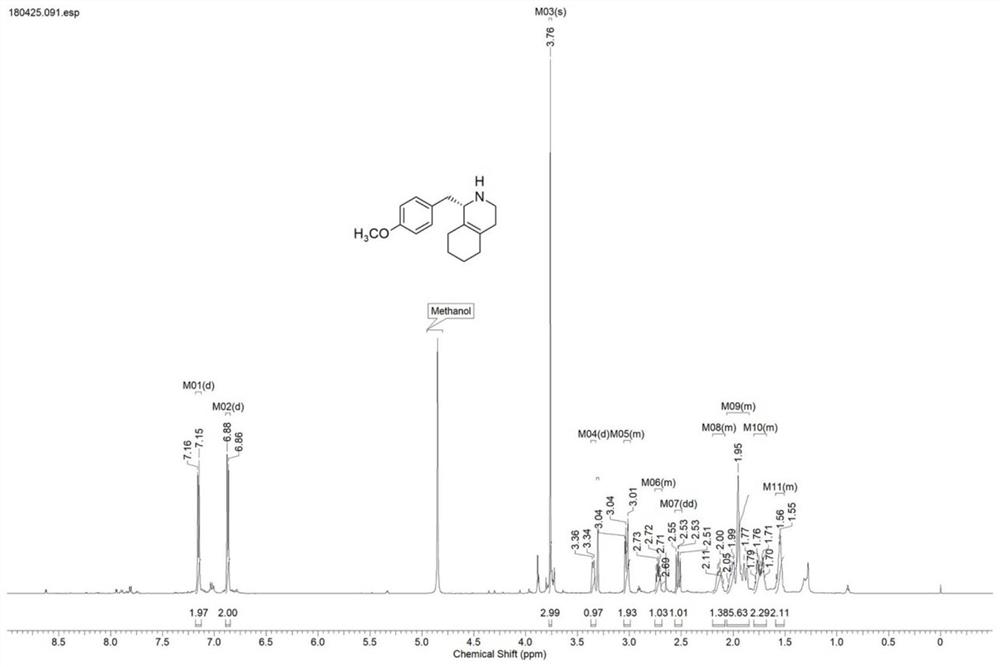
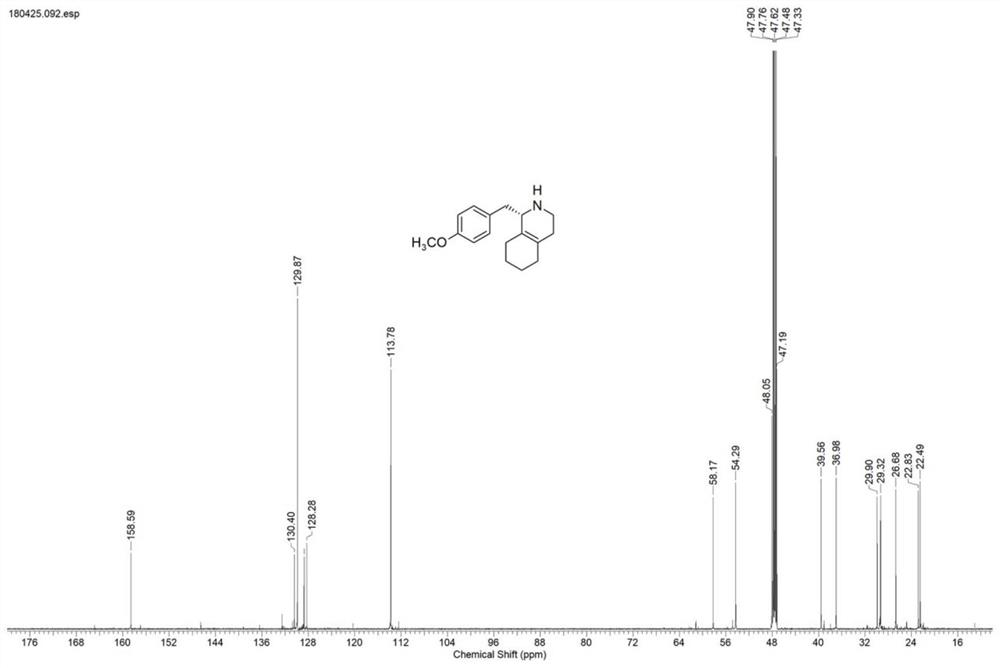
[0030] Take 2.5g of IR1 resting cells and resuspend in 100mL sodium phosphate buffer (100mM, pH 7.5), add glucose (1g), NADP + (5mg), GDH enzyme powder (50mg), 1-(4-methoxybenzyl)-3,4,5,6,7,8-hexahydroisoquinoline hydrochloride (1) (0.5g) , use 10% sodium carbonate solution to control pH 7.5, react under nitrogen protection at 25°C for 24 hours, HPLC detection shows that the reaction is complete (liquid chromatography column: Daicel CHIRALPAK OJ-H (250mm×4.6mm, 5μm), mobile phase: iso Propanol (0.5% ethanolamine) / n-hexane=10:90, detection wavelength: 230nm, flow rate: 0.5mL / min), 10% sodium carbonate solution to adjust pH to 9.0, ethyl acetate extraction (100mL*3), anhydrous After drying with sodium sulfate, it was spin-dried, separated and purified by column chromatography to obtain (S)-1-(4-methoxybenzyl)-1,2,3,4,5,6,7,8-octahydroisoquine The morphine ((S)-...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap