Method for detecting adverse reaction signals of combined medication
A technology of adverse reactions and drug combination, applied in the direction of drug reference, etc., can solve the problems of difficulty and limitation in obtaining drug-protein interaction data
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
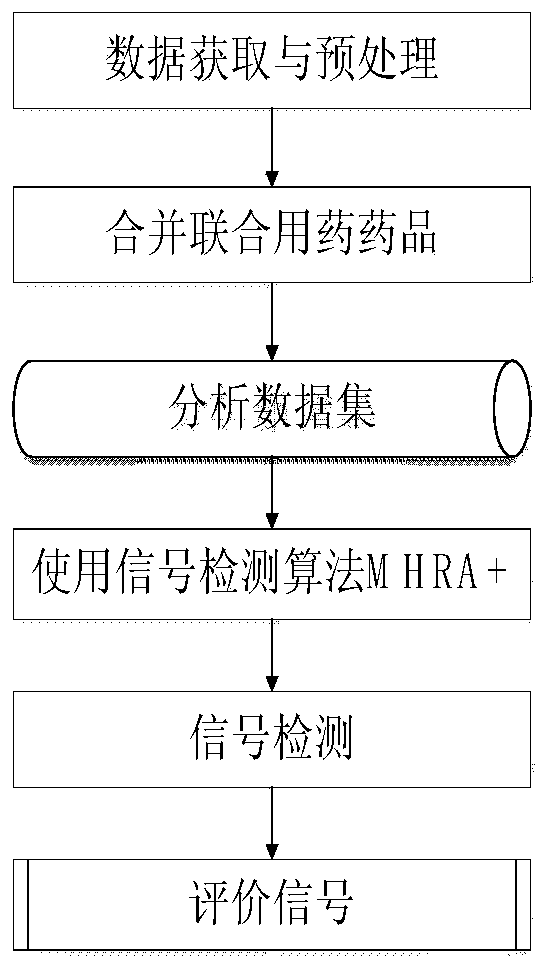
[0044] The present invention discloses a method for detecting signals of adverse reactions of drug combination, comprising the following steps:
[0045] S1. Raw data acquisition and processing steps:
[0046] Step 1.1) Data acquisition: There are 1,823,144 ADR reports in the National Adverse Drug Reaction Testing Center from January 1, 2010 to December 31, 2011, including 879,109 single drug reports and 330,235 combined drug reports. Using Microsoft Visual FoxPro software to select 183 kinds of antipsychotic and antiepileptic drugs, of which 1,707 records of combined drug data include 1,159 combined drug combinations, and 1,698 single drug data records include 36 drugs and 393 adverse reactions. Accounting for 50.13% of the total data. In this example, western medicine data is selected as the experimental object.
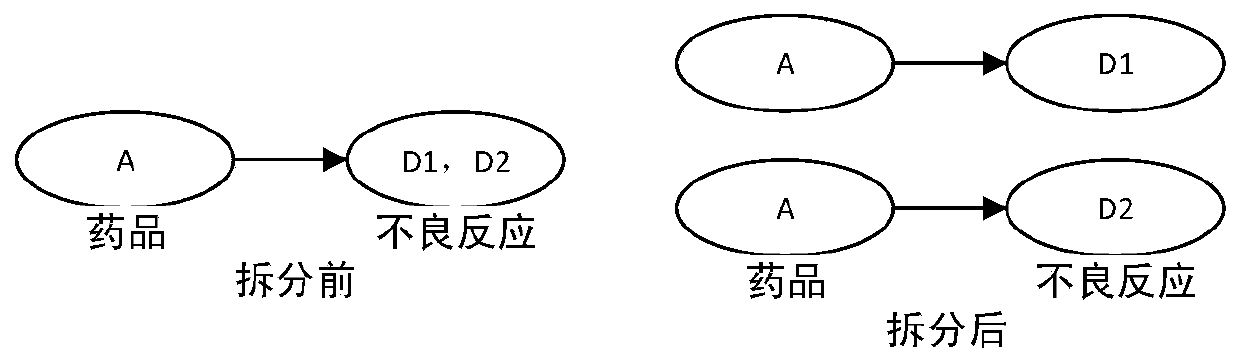
[0047] Step 1.2) Data processing: re-normalize the inconsistent data between the drug name and the adverse reaction name in the single drug and combined drug data...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com