Novel symmetric purpurine compound and preparation method and applications thereof
A compound and symmetrical technology, applied in the field of novel symmetrical viologen compounds and their preparation, to achieve the effects of good electron accepting ability, good electron-acquiring performance and easy adjustment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
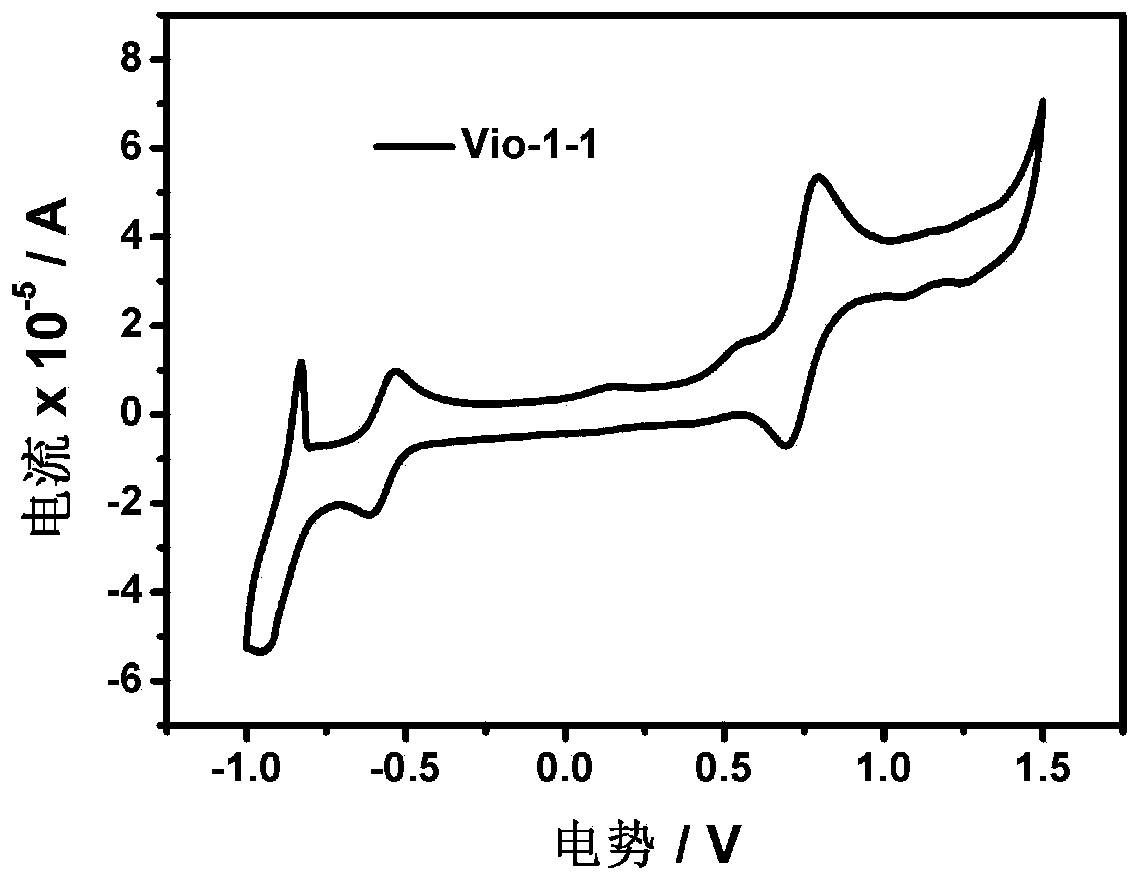
[0042] Embodiment 1: the preparation of Vio-1-1 and Vio-2-1
[0043]
[0044] (1) Preparation of compound 5: 4,4'-bipyridine (154mg, 1mmol) and 1-chloro-2,4-dinitrobenzene (707mg, 3.5mmol) were refluxed in acetonitrile or ethanol solvent for 24h, cooled Return to room temperature, filter with suction, wash with acetonitrile three times, then wash with ether three times, and dry in vacuum to obtain compound 5. Yield: 74%. 1 H NMR (400MHz,D 2 O) δ(ppm): 9.41(d, J=8Hz, 2H), 9.35(d, J=8Hz, 2H), 8.88(m, 6H), 8.24(d, J=8Hz, 2H). 13 C NMR (100MHz,D 2 O) δ (ppm): 150.7, 148.8, 143.9, 139.9, 134.4, 133.2, 132.6, 123.7, 121.0.
[0045] (2) Preparation of Compound 3: Compound 1, bis(4-tert-butylphenyl)amine (281mg, 1mmol) and sodium hydride (60% in mineral oil) (80mg, 1mmol) in anhydrous DMSO solvent at room temperature Stirred at low temperature for 30min, then added compound 2, p-fluoronitrobenzene (141mg, 1mmol), stirred at 150°C for 18h; cooled to room temperature, poured the...
Embodiment 2
[0053] Embodiment 2: The specific operation steps of the electrochromic and electrochromic light-emitting dual-function devices made of Vio-1-1 or Vio-2-1 are as follows:
[0054] (1) Dissolve Vio-1-1 or Vio-2-1 and polyvinylpyrrolidone (PVP) with a mass ratio of 1:1 in a low-boiling organic solvent, drop-coat it on ITO glass and dry it in a vacuum at 80°C Box drying for 12 hours;
[0055] (2) Dissolve polymethyl methacrylate (PMMA), lithium perchlorate, and propylene carbonate (PC) in a low-boiling point organic solvent, and drop-coat it on the ITO glass with the material coated in (1). The ITO glass is overlapped and dried in a vacuum oven at 80°C for 12 hours to bond the two pieces of ITO glass together.
Embodiment 3
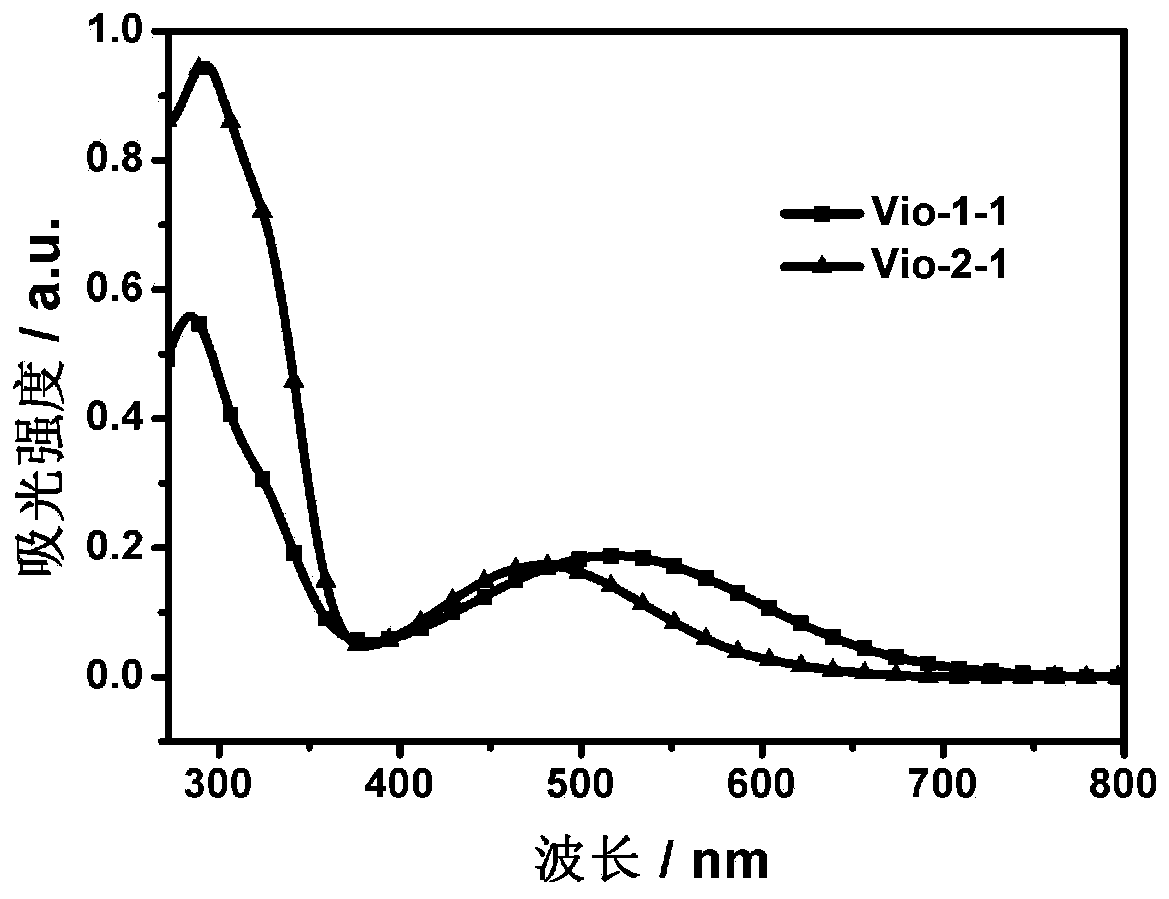
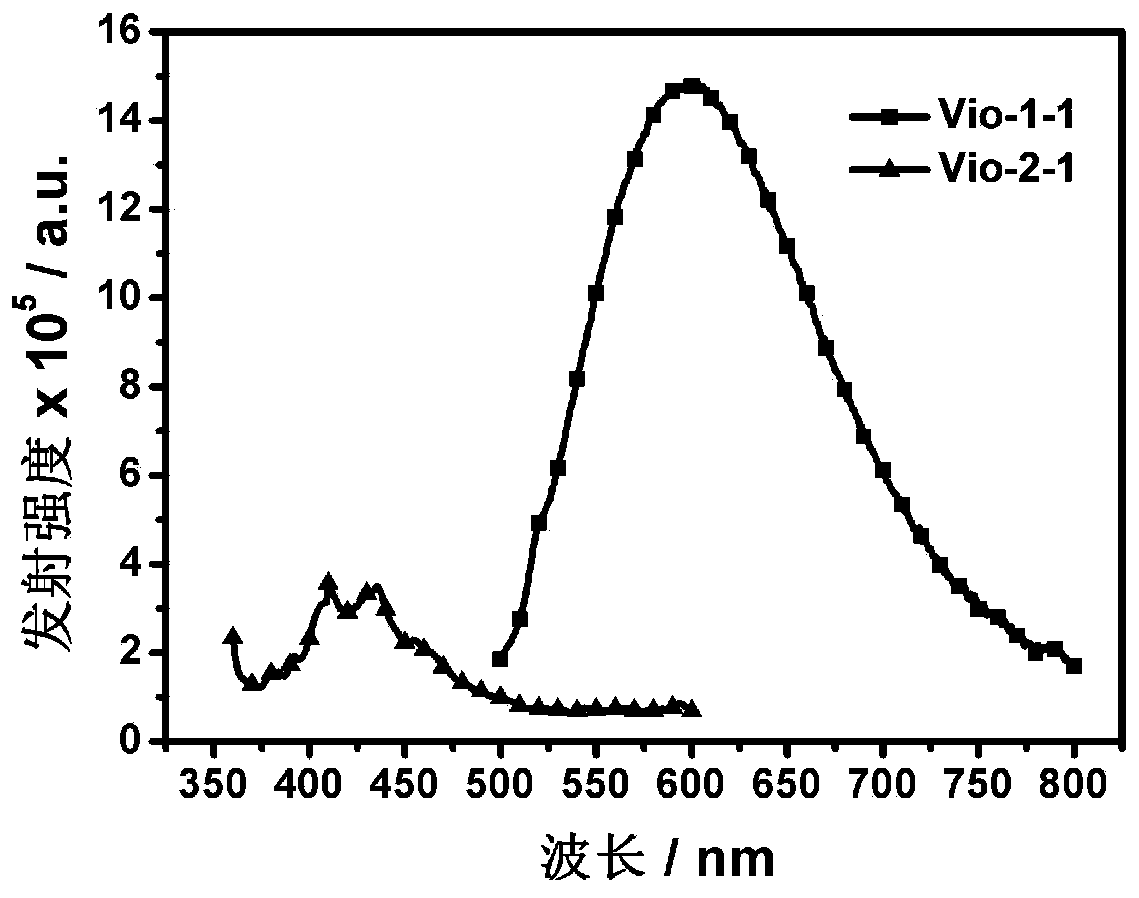
[0056]Embodiment 3: Absorption and emission spectrum test of Vio-1-1 and Vio-2-1
[0057] The spectrum test concentration used in the present invention is 10 μM, and the test solvent is acetonitrile. When measuring the emission spectrum, the excitation wavelength of Vio-1-1 is 405nm, and the excitation wavelength of Vio-2-1 is 350nm.
[0058] Absorption and emission spectra of Vio-1-1 and Vio-2-1 as figure 1 and figure 2 shown. The absorption peak around 300nm is the characteristic absorption peak of viologen. The maximum absorption peak of Vio-1-1 is around 520nm, while the maximum absorption peak of Vio-2-1 is around 480nm; The emission peak is around 425nm and emits green light. This is caused by the difference in the strength of the charge transfer ability in the two molecules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Excitation wavelength | aaaaa | aaaaa |
| Excitation wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com