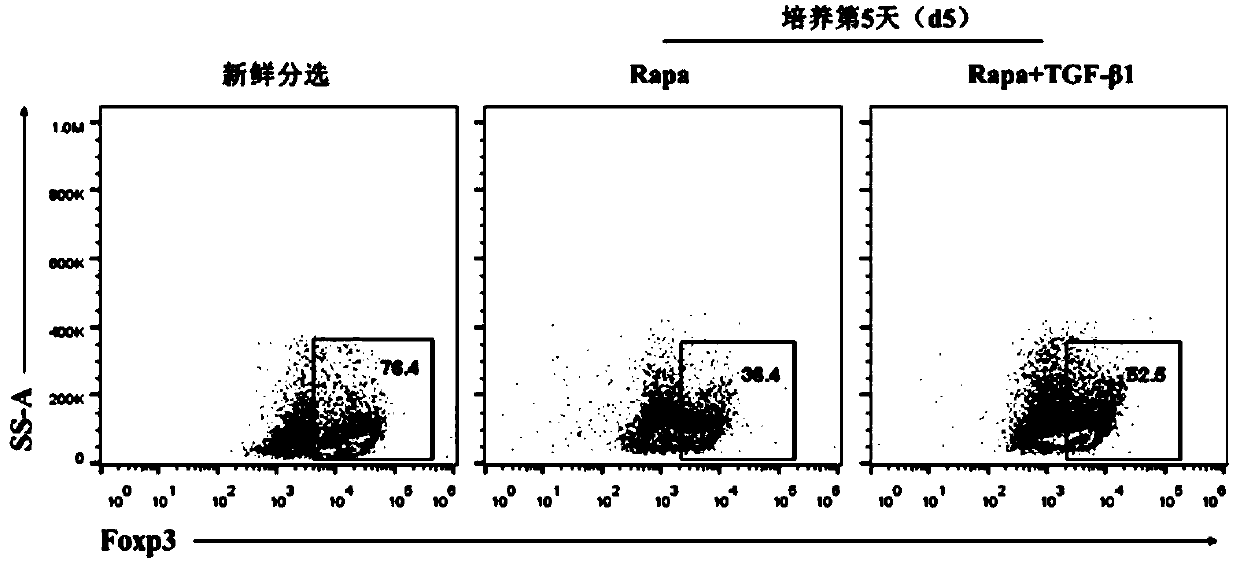
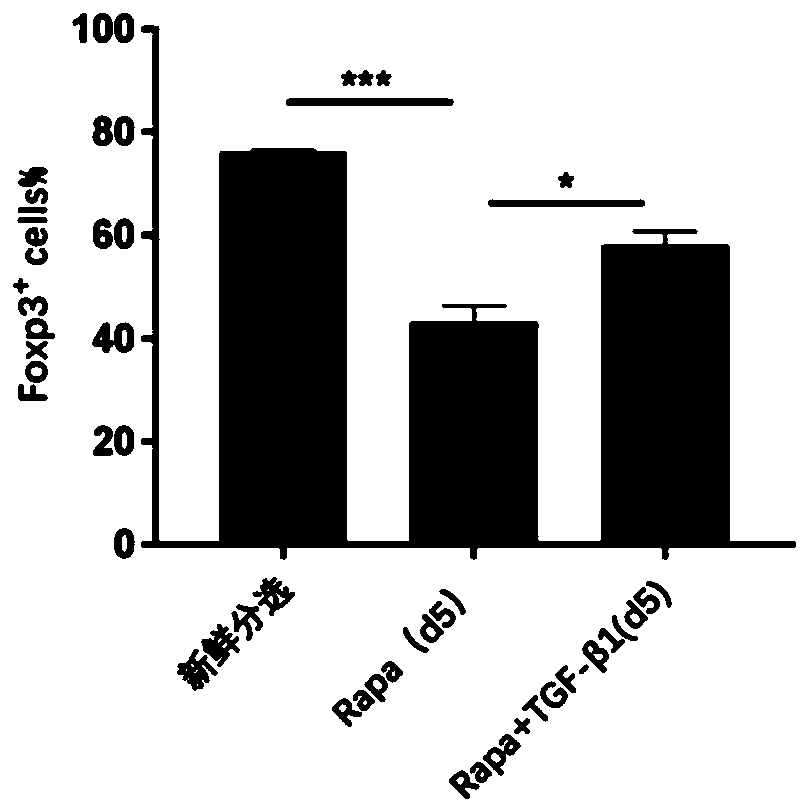
Primary regulatory T cell in-vitro amplification system and method
An in vitro expansion and expansion system technology, which is applied in the in vitro expansion system and expansion field of primary regulatory T cells, can solve the problems of reducing the purity of regulatory T cells, residual T cells, and limited practical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0040] The preparation of preparation example 1 basal medium
[0041]In a sterile operating bench, add 10ml of fetal bovine serum, 1ml of 100* double antibody (penicillin + streptomycin), 11mg of sodium pyruvate, 3% glutamine (using a micrometer with a pore size of 0.22μm) to the 1640 medium (100ml). Pore membrane filtration sterilization) 1ml, β-mercaptoethanol 0.39mg, fully shake and shake, and prepare the basal medium.
Embodiment 1
[0042] The preparation of embodiment 1 in vitro amplification system
[0043] On the basis of the basal medium obtained in Preparation Example 1, 0.2 mg of anti-CD3 / 28Ab (final concentration 2 μg / ml), 0.1 ml of mIL-2 (1 million units / ml) (final concentration 1000 U / ml), Rapa 10 μg (final concentration 100 ng / ml), TGF-β1 0.4 μg (final concentration 4 ng / ml), shake well, and make regulatory T cell in vitro expansion system.
Embodiment 2
[0044] The preparation of embodiment 2 in vitro amplification system
[0045] The preparation method and raw materials are as in Example 1, except that the amount of Rapa added is 15 μg, and the final concentration is 150 ng / ml, and the amount of TGF-β1 added is 0.5 μg, and the final concentration is 5 ng / ml.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap