Modified ube3a gene for a gene therapy approach for angelman syndrome
A composition and sequence technology, applied in gene therapy, chemical instruments and methods, biochemical equipment and methods, etc., can solve problems such as preventing the overall distribution of proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
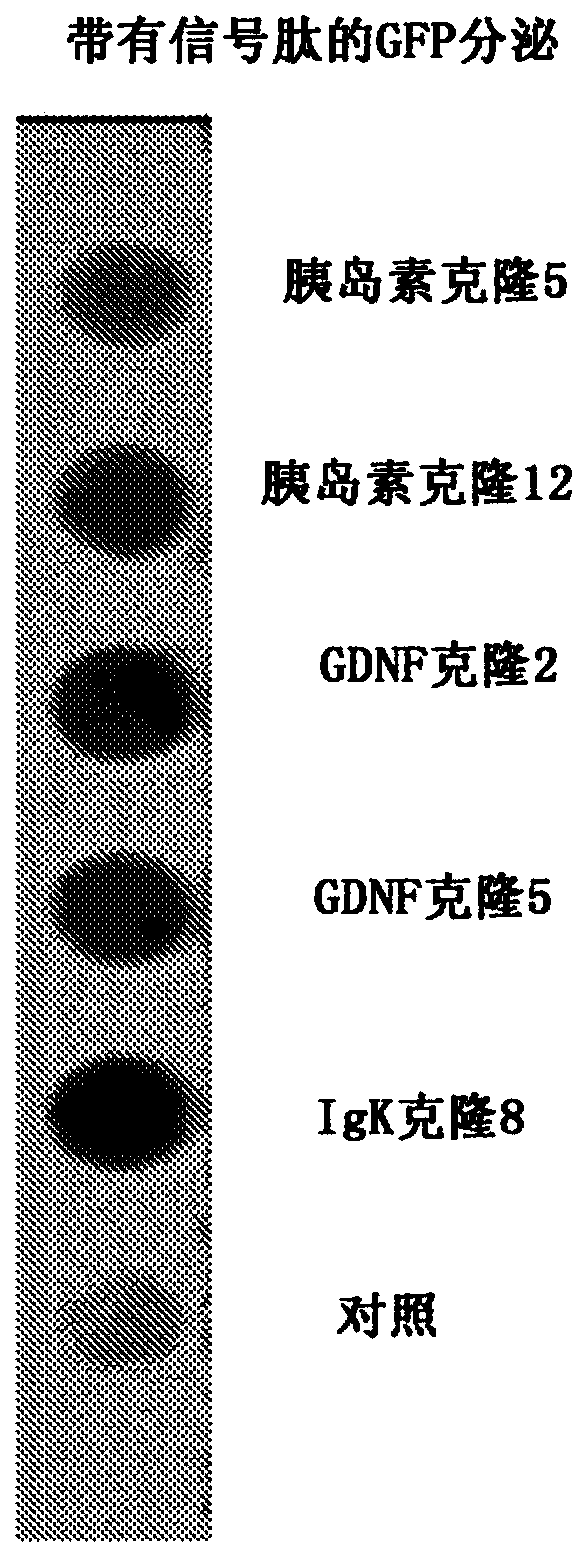
[0095] Example 1 - Efficiency of secretion signaling
[0096] To test the efficacy of the secretion signal, GFP (SEQ ID No: 1 ) (XM 013480425.1 ) was cloned in frame with human insulin, GDNF (SEQ ID No: 2) (AB675653.1 ) or the IgK signal peptide.
[0097] ATGGCTCGTC TTTCTTTTGT TTCTCTTCTT TCTCTGTCAC TGCTCTTCGG GCAGCAAGCAGTCAGAGCTC AGAATTACAC CATGGTGAGC AAGGGCGAGG AGCTGTTCAC CGGGGTGGTG CCCATCCTGGTCGAGCTGGA CGGCGACGTA AACGGCCACA AGTTCAGCGT GTCCGGCGAG GGCGAGGGCG ATGCCACCTACGGCAAGGAC TGCCTGAAGT TCATCTGCAC CACCGGCAAG CTGCCCGTGC CCTGGCCCAC CCTCGTGACCACCTTCGGCT ACGGCCTGAT GTGCTTCGCC CGCTACCCCG ACCACATGAA GCAGCACGAC TTCTTCAAGTCCGCCATGCC CGAAGGCTAC GTCCAGGAGC GCACCATCTT CTTCAAGGAC GACGGCAACT ACAAGACCCGCGCCGAGGTG AAGTTCGAGG GCGACACCCT GGTGAACCGC ATCGAGCTGA AGGGCATCGA CTTCAAGGAGGACGGCAACA TCCTGGGGCA CAAGCTGGAG TACAACTACA ACAGCCACAA CGTCTATATC ATGGCCGACAAGCAGAAGAA CGGCATCAAG GTGAACTTCA AGATCCGCCA CAACATCGAG GACGGCAGCG TGCAGCTCGCCGACCACTAC CAGCAGAACA CCCCCATCGG CGACGGCCCC GTGCTGCTGC CCGACAAC...
Embodiment 2
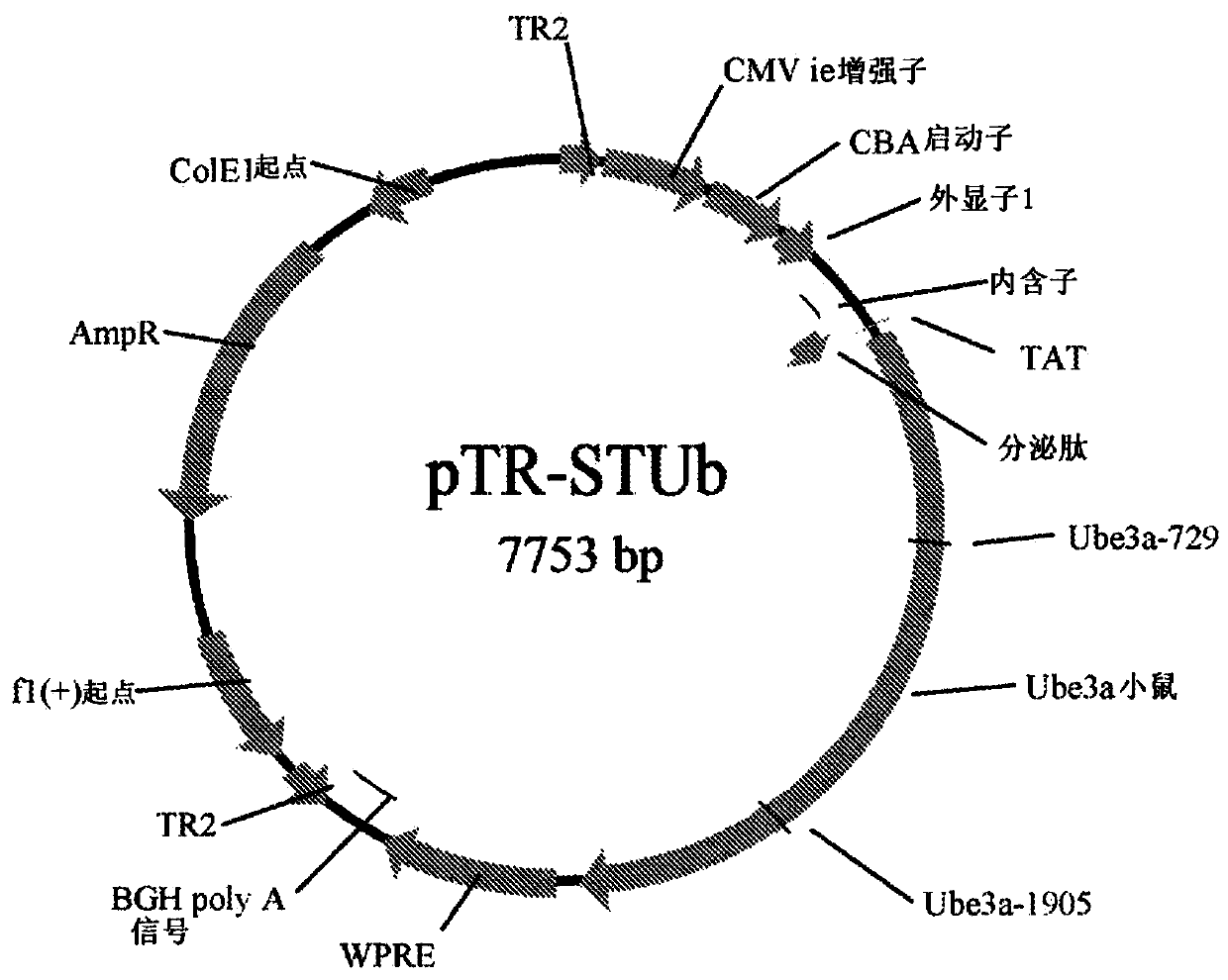
[0105] Example 2 - Mouse UBE3A Vector Constructs
[0106] The pTR plasmid was used to generate mouse UBE3A vector constructs. The mouse (Mus musculus) UBE3A gene is formed by cDNA (U82122.1);
[0107] ATGAAGCGAG CAGCTGCAAA GCATCTAATA GAACGCTACT ACCATCAGTT AACTGAGGGCTGTGGAAATG AGGCCTGCAC GAATGAGTTT TGTGCTTCCT GTCCAACTTT TCTTCGTATG GATAACAATGCAGCAGCTAT TAAAGCCCTT GAGCTTTATA AAATTAATGC AAAACTCTGT GATCCTCATC CCTCCAAGAAAGGAGCAAGC TCAGCTTACC TTGAGAACTC AAAAGGTGCA TCTAACAACT CAGAGATAAA AATGAACAAGAAGGAAGGAA AAGATTTTAA AGATGTGATT TACCTAACTG AAGAGAAAGT ATATGAAATT TATGAATTTTGTAGAGAGAG TGAGGATTAT TCCCCTTTAA TTCGTGTAAT TGGAAGAATA TTTTCTAGTG CTGAGGCACTGGTTCTGAGC TTTCGGAAAG TCAAACAGCA CACAAAGGAG GAATTGAAAT CTCTTCAAGA AAAGGATGAAGACAAGGATG AAGATGAAAA GGAAAAAGCT GCATGTTCTG CTGCTGCTAT GGAAGAAGAC TCAGAAGCATCTTCTTCAAG GATGGGTGAT AGTTCACAGG GAGACAACAA TGTACAAAAA TTAGGTCCTG ATGATGTGACTGTGGATATT GATGCTATTA GAAGGGTCTA CAGCAGTTTG CTCGCTAATG AAAAATTAGA AACTGCCTTCCTGAATGCAC TTGTATATCT GTCACCTAAC GTGGAATG...
Embodiment 3
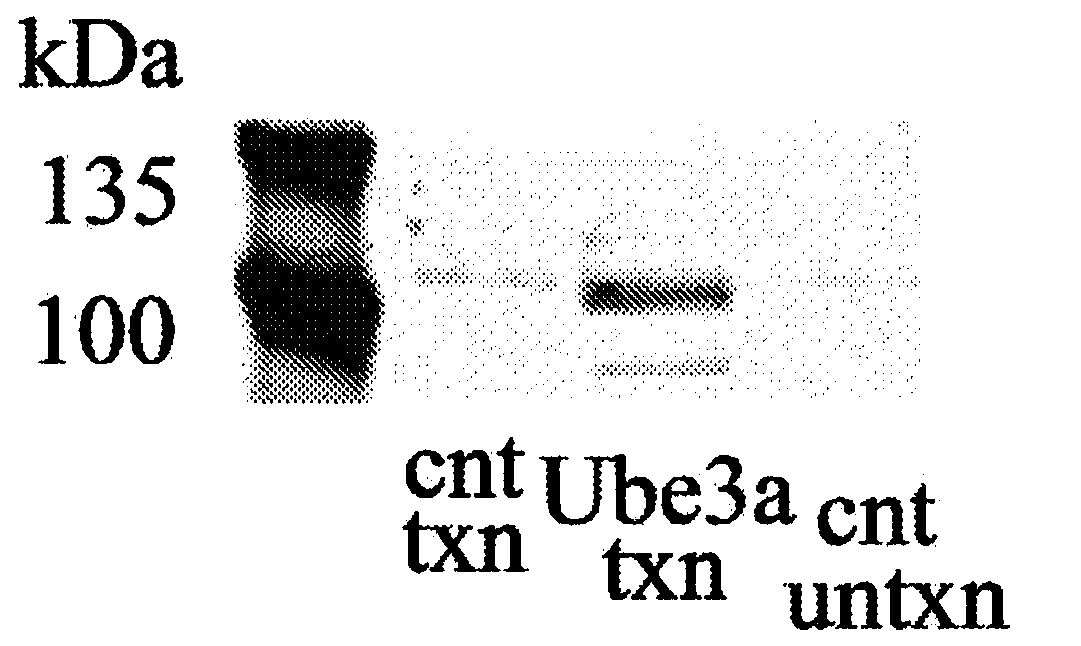
[0116] Example 3 - In Vitro Testing of Mouse UBE3A Vector Constructs
[0117] The mouse vector properties of the constructs generated in Example 2 were tested in HEK293 cells (American Type Culture Collection, Manassas, VA). HEK293 cells were cultured in Dulbecco's modified essential medium (DMEM) containing 10% FBS and 1% penicillin / streptomycin at 37°C in 5% CO 2 Grow and subculture at 80% confluency.
[0118] The vector (2 μg / well in a 6-well plate) was transfected into cells using the PEI transfection method. Two days before transfection, cells were incubated with 0.5 × 10 6 Cells / well were subcultured in DMEM medium in 6-well plates. The medium was changed the night before transfection. Endotoxin-free dH 2 O was heated to 80°C and polyethyleneimine (Sigma-Aldrich Co. LLC, St. Louis, MO) was dissolved. The solution was cooled to about 25°C, and the solution was neutralized with sodium hydroxide. For each transfection well, add AAV4-STUb vector or negative control (m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com