Modified UBE3A gene for a gene therapy approach for angelman syndrome
A genome, seqidno.13 technology, applied in gene therapy, chemical instruments and methods, biochemical equipment and methods, etc., can solve problems such as unsolved diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
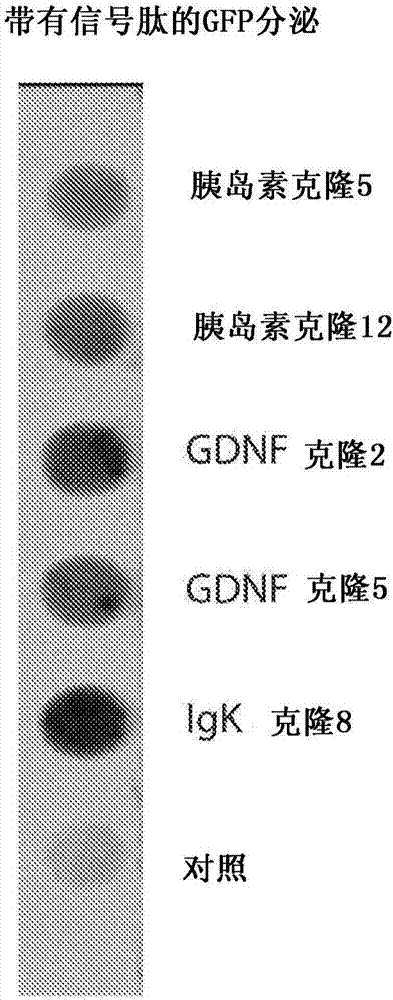
[0057] To test the efficacy of the secretion signal, GFP was cloned in frame with human insulin, GDNF or IgK signal peptides. The construct was inserted into pTR plasmid and transfected into HEK293 cells (American Type Culture Collection, Manassas, VA). HEK293 cells were cultured in Dulbecco's Modified Essential Medium (DMEM) containing 10% FBS and 1% Pen / Strep at 37°C in 5% CO 2 Grow and subculture at 80% confluency.
[0058] The vector (2 μg / well in a 6-well plate) was transfected into the cells using the PEI transfection method. Two days before transfection, the cells were plated in 6-well plates with DMEM medium at 0.5 x 10 6 cells / well subcultured. Change the medium the night before transfection. Endotoxin-free dH 2 O was heated to about 80°C and polyethyleneimine (Sigma-Aldrich Co. LLC, St. Louis, MO) was dissolved. The solution was allowed to cool to about 25°C, and the solution was neutralized with sodium hydroxide. For each transfected well, add AAV4-STUb vecto...
Embodiment 2
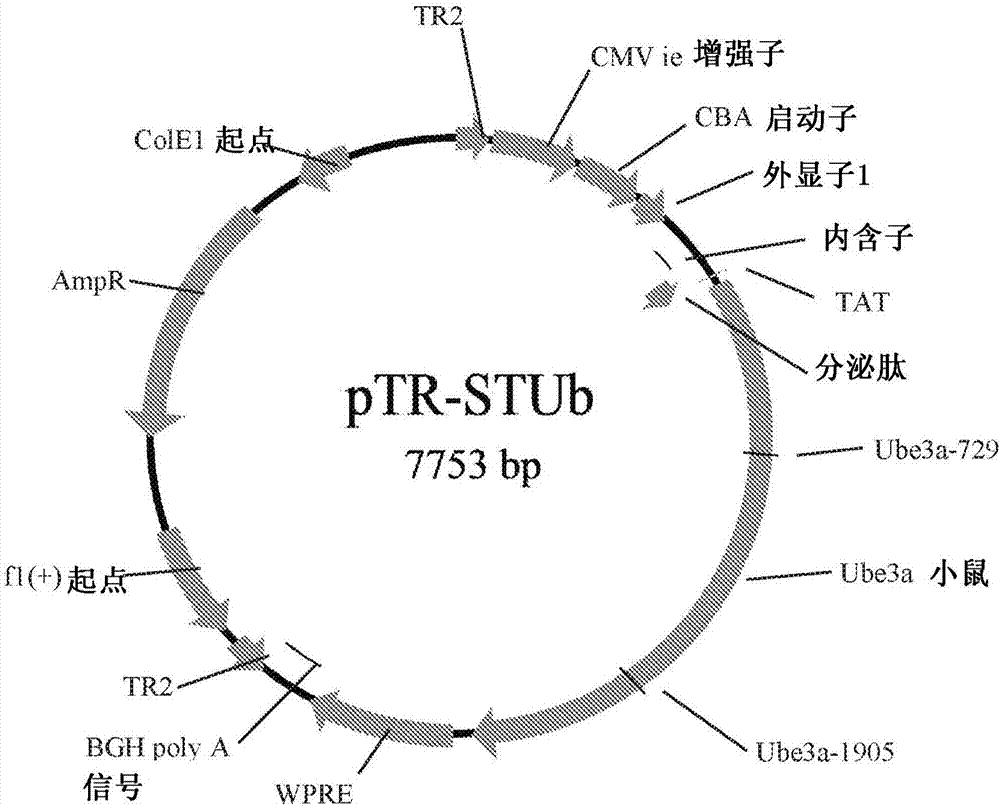
[0062] The mouse-UBE3A vector construct was generated using the pTR plasmid. The mouse (Mus musculus) UBE3A gene was formed from the following cDNA (U82122.1):
[0063]
[0064]
[0065] The cDNA was subcloned and sequenced. The mouse UBE3A gene (SEQ ID No. 1) was fused to the DNA sequence (SEQ ID No. 2) encoding the secretion signal peptide and the HIV TAT sequence (SEQ ID No. 4). The secretion signal peptide has the DNA sequence:
[0066] atg gcc ctg ttg gtg cac ttc cta ccc ctg ctg gcc ctg ctt gcc ctc tgggag ccc aaa ccc acc cag gct ttt gtc (SEQ ID No.2), its encoded protein sequence:
[0067] MALLVHFLPLLALLALWEPKPTQAFV (SEQ ID No. 3);
[0068] At the same time the HIV TAT sequence is:
[0069] tac ggc aga aag aag agg agg cag aga agg aga (SEQ ID No.4), its encoded protein sequence:
[0070] YGRKKRRQRRR (SEQ ID No. 5).
[0071] The construct sequence of SEQ ID No. 1 fused to SEQ ID No. 2 and SEQ ID No. 4 was inserted into the pTR plasmid. The plasmid was cleaved w...
Embodiment 3
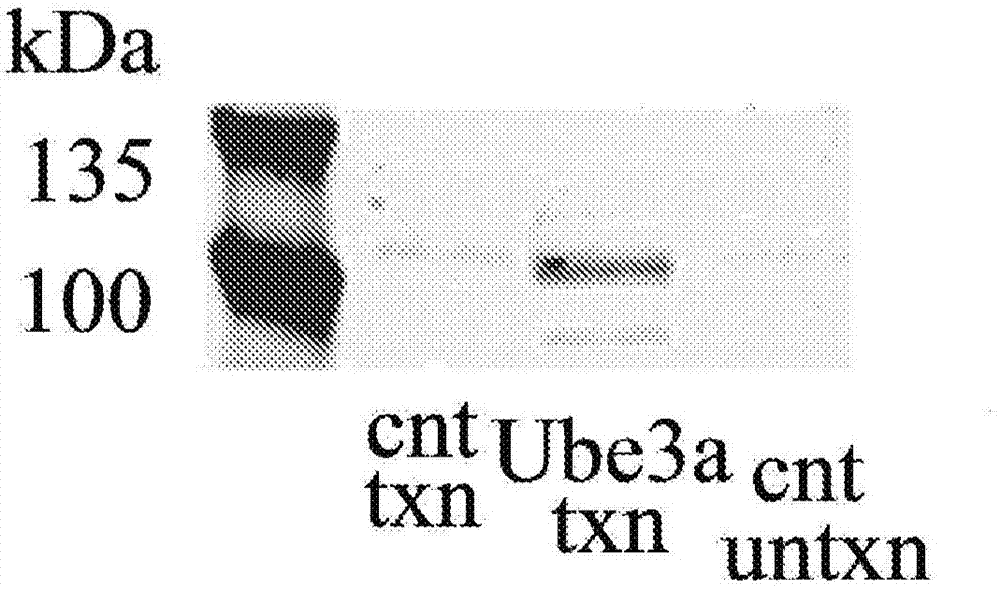
[0074] The mouse vector properties of the constructs generated in Example 2 were tested in HEK293 cells (American Type Culture Collection, Manassas, VA). HEK293 cells were cultured in Dulbecco's Modified Essential Medium (DMEM) containing 10% FBS and 1% Pen / Strep at 37°C in 5% CO 2 Grow and subculture at 80% confluency.
[0075] The vector (2 μg / well in a 6-well plate) was transfected into the cells using the PEI transfection method. Two days before transfection, the cells were plated in a 6-well plate at 0.5 x 10 6 cells / well were subcultured in DMEM medium. Change the medium the night before transfection. Endotoxin-free dH 2 O was heated to about 80°C and polyethyleneimine (Sigma-Aldrich Co. LLC, St. Louis, MO) was dissolved. The solution was allowed to cool to about 25°C, and the solution was neutralized with sodium hydroxide. For each transfected well, add AAV4-STUb vector or negative control (medium only) at 2 μg per 200 μL in serum-free DMEM, and add 9 μL of 1 μg / μ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com