Scaffold proteins
A technology of heterologous peptides and amino acids, applied in the field of scaffold proteins, can solve problems such as no regulation or change of thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1339] Example 1 - Mutations can be independently combined to modulate stability
[1340] The following mutations were made relative to SEQ ID NO: 1:
[1341]
[1342] These all increased the Tm of hSteA Y35W.
[1343] This suggests that the order of mutations does not matter. In other words, this demonstrates that stability (eg, Tm) modulating mutations taught herein can be independently made or independently combined.
[1344] An advantage of the present invention is that the specific stability-modulating mutations taught herein are independent of each other to achieve their respective effects.
Embodiment 2
[1345] Example 2: Heterologous Peptide Inserts
[1346] Testing the heterologous peptide GGSGGSGGS inserted into L2 and L4
[1347] 3 different positions per ring (9 combinations)
[1348] Measuring Thermal Stability on Optim 2
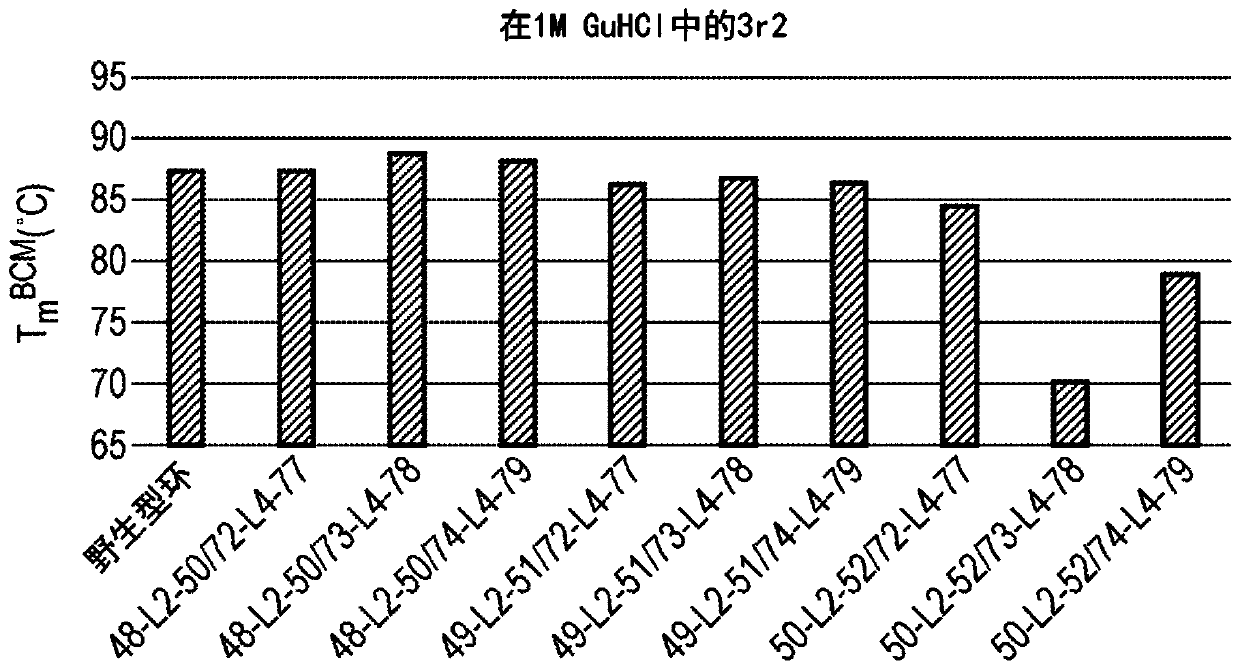
[1349] 3r2 measured without denaturant but stability out of range
[1350] 3r2 was measured again in the presence of 1M GuHCl to bring the Tm in range
[1351] 3t4 was measured in the presence of SYPRO Orange since there is no intrinsic tryptophan fluorescence in this peptide
[1352] In summary, the options for various examples are shown below:
[1353] SQT (SEQ ID NO: 24 of WO 2009 / 136182)
[1354]
[1355] 3r2 (SEQ ID NO: 19)
[1356]
[1357] 3t4 (SEQ ID NO: 23)
[1358]
[1359] 3r2 (SEQ ID NO: 19) / t4 (SEQ ID NO: 23)
[1360]
[1361] 3r2 (SEQ ID NO: 19) / t4 (SEQ ID NO: 23)
[1362]
[1363] 3r2 (SEQ ID NO: 19) / t4 (SEQ ID NO: 23)
[1364]
[1365] The result is as figure 1 shown.
[1366] This indicates that insertio...
Embodiment 3
[1374] Example 3: Exemplary Scaffold Proteins
[1375] Exemplary scaffold proteins are shown.
[1376] In this experiment, Tm was measured by DSC (r / t) and Optim 2 (3r).
[1377] Exemplary holders for research applications:
[1378] 3r1-hSteA Y35W N32G V48D M65I Q42E T51L (A59VΔD61) (E29K K30E E33K) (SEQ ID NO: 18)
[1379] 3r2-hSteA Y35W N32G V48D M65I Q42E T51L (A59V G60NΔD61N62G) (E29K K30EE33K) (SEQ ID NO: 19)
[1380] Exemplary stents for therapeutic applications:
[1381] SEQ ID NO:20 3t1-hSteA N32G V48D
[1382] SEQ ID NO:21 3t2–hSteA N32G V48D M65I
[1383] SEQ ID NO:22 3t3–hSteA N32G V48D M65I T51L
[1384] SEQ ID NO:23 3t4–hSteA N32G V48D M65I Q42E
[1385] SEQ ID NO:24 3t5–hSteA N32G V48D M65I Q42E T51L
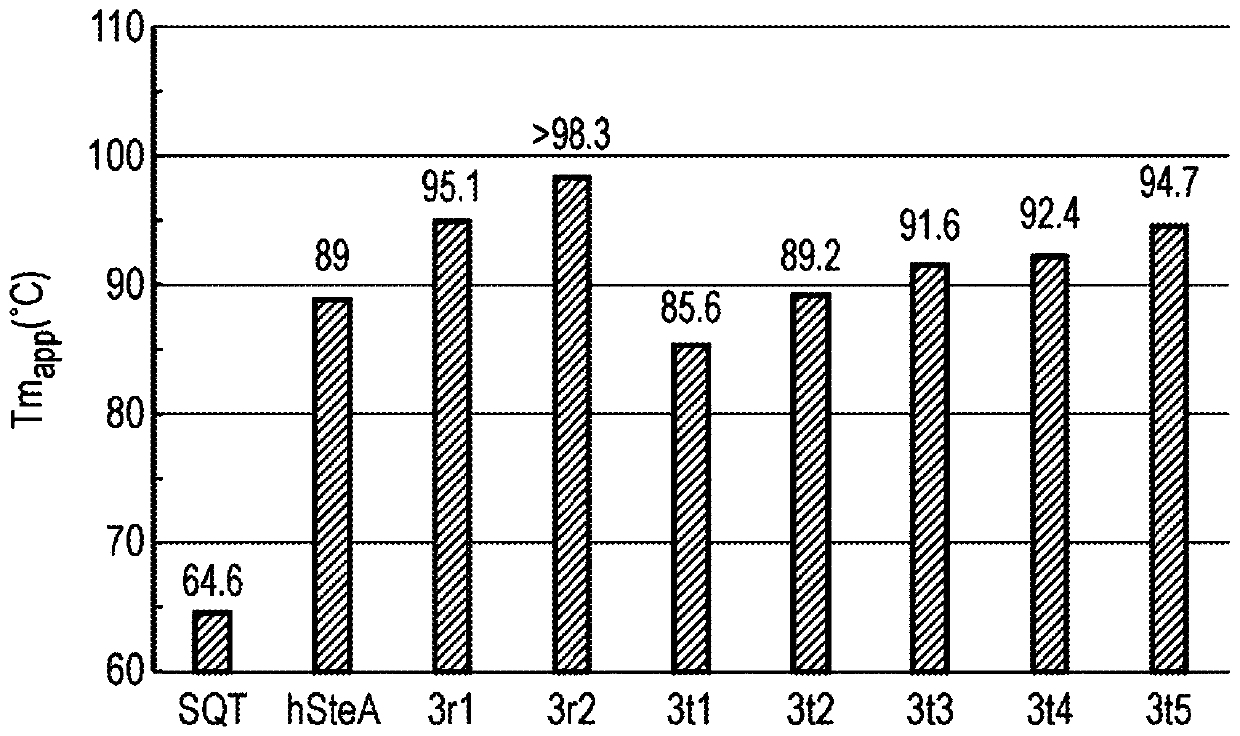
[1386] figure 2 Data are shown for Tm as a measure of thermal stability.
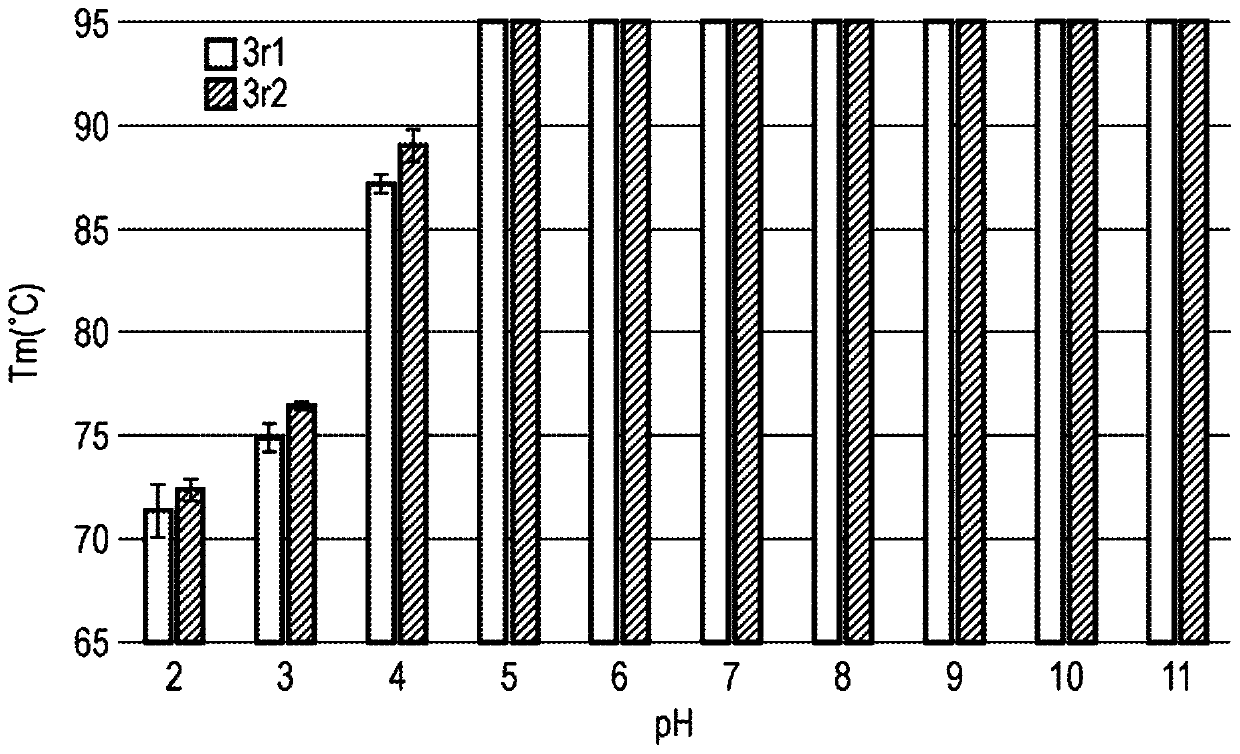
[1387] image 3 Tm measured at different pH values are shown.
[1388] Figure 4 shows the CD spectrum.
[1389] We generated DSC data for the above exemplary scaffolds and ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal stability | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap