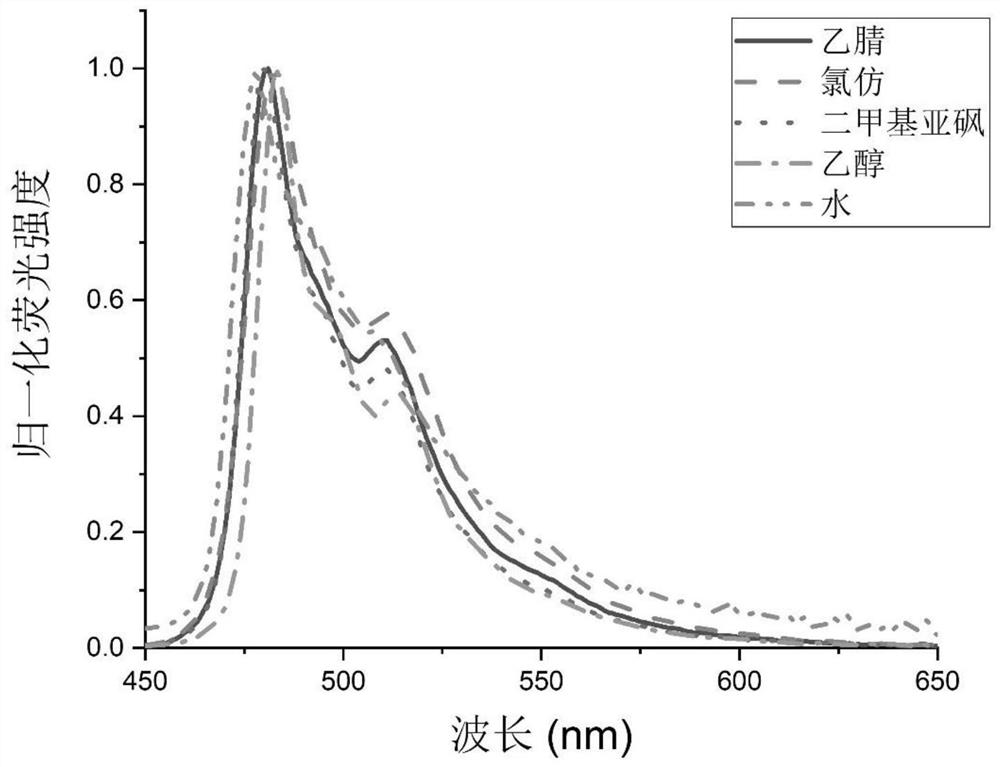
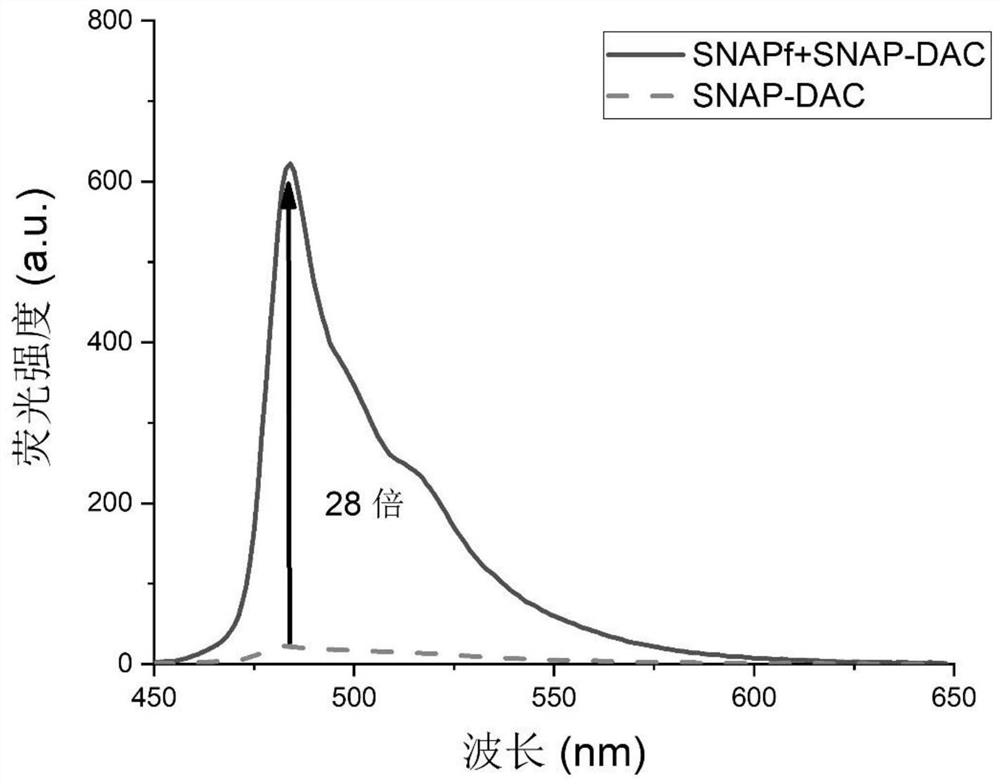
A kind of high brightness, high stability wash-free snap-tag probe and its preparation method and application
A high-stability, high-brightness technology, applied in the field of fluorescent probes, which can solve the problems of fluorescence signal error, fluorescence accompanied by blue shift, and capture of fluorescence signal, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
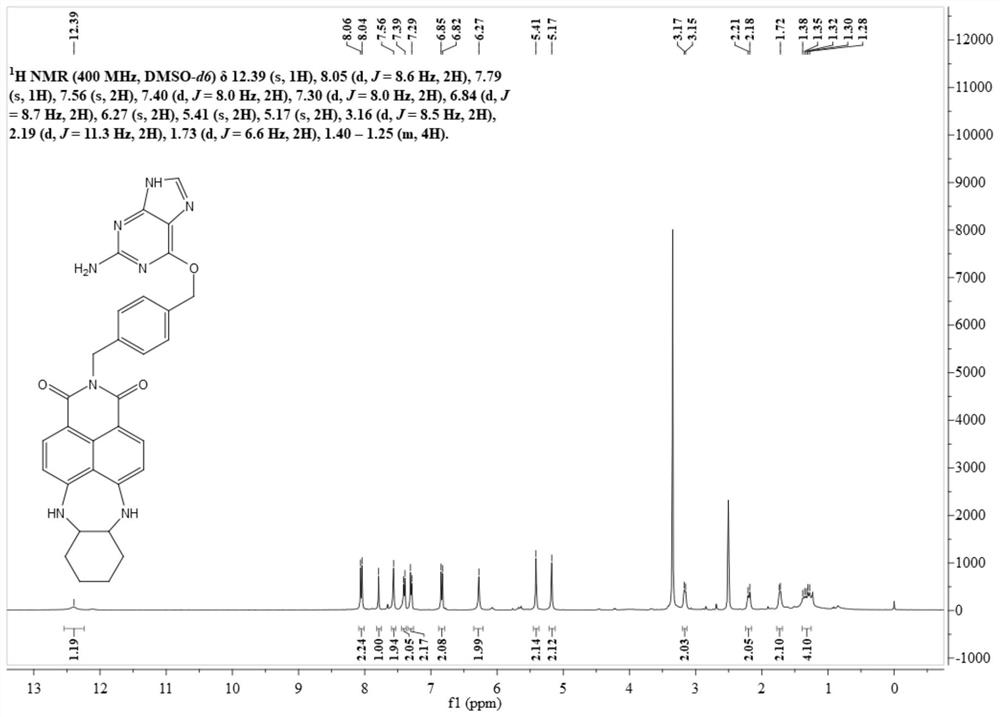
[0044] Synthesis of Intermediate N-(4-Hydroxymethyl)benzyl-4-bromo-5-nitro-1,8-naphthalimide (BA-NBr)
[0045]
[0046] 4-Bromo-5-nitro-1,8-naphthalimide (1.00 g, 3.11 mmol) was dissolved in 50 mL of ethanol, and 4-aminomethylbenzyl alcohol (853 mg, 6.22 mmol) was added thereto. After 10 h at 80°C, the solvent was distilled off under reduced pressure, and the residue was separated through a silica gel column (petroleum ether: dichloromethane = 3:1-dichloromethane: methanol = 200:1, V / V) to obtain 480 mg of off-white solid. Yield 35%. Its nuclear magnetic spectrum hydrogen spectrum data are as follows:
[0047] 1 H NMR (400MHz, DMSO-d 6 )δ8.69(d, J=8.1Hz, 2H), 8.50–8.39(m, 2H), 7.35(d, J=8.1Hz, 2H), 7.25(d, J=7.9Hz, 2H), 5.23( s,2H),5.13(t,J=5.8Hz, 1H),4.45(d,J=5.5Hz,2H).
[0048] Synthesis of BA-DAC
[0049]
[0050] BA-NBr (200 mg, 0.45 mmol) was dissolved in 30 mL of ethylene glycol methyl ether, and 400 mg of 1,2-cyclohexanediamine was added thereto. The reaction...
Embodiment 2
[0063] Synthesis of Intermediate N-(4-Hydroxymethyl)benzyl-4-bromo-5-nitro-1,8-naphthalimide (BA-NBr)
[0064]
[0065] 4-Bromo-5-nitro-1,8-naphthalimide (1.00 g, 3.11 mmol) was dissolved in 20 mL of ethanol, and 4-aminomethylbenzyl alcohol (500 mg, 3.65 mmol) was added thereto. After 10 h at 80°C, the solvent was distilled off under reduced pressure, and the residue was separated through a silica gel column (petroleum ether: dichloromethane=3:1-dichloromethane:methanol=200:1, V / V) to obtain 480 mg of off-white solid. Yield 35%.
[0066] Synthesis of BA-DAC
[0067]
[0068] BA-NBr (200 mg, 0.45 mmol) was dissolved in 10 mL of ethylene glycol methyl ether, and 200 mg of 1,2-cyclohexanediamine was added thereto. The reaction solution was slowly heated to 110°C and reacted for 12h. Ethylene glycol methyl ether was removed under reduced pressure, and the residue was separated through a silica gel column (dichloromethane:methanol=80:1, V / V) to obtain 90 mg of a yellow sol...
Embodiment 3
[0075] Synthesis of Intermediate N-(4-Hydroxymethyl)benzyl-4-bromo-5-nitro-1,8-naphthalimide (BA-NBr)
[0076]
[0077] 4-Bromo-5-nitro-1,8-naphthalimide (1.00 g, 3.11 mmol) was dissolved in 80 mL of ethanol, and 4-aminomethylbenzyl alcohol (2 g, 14.6 mmol) was added thereto. After 10 h at 90°C, the solvent was distilled off under reduced pressure, and the residue was separated through a silica gel column (petroleum ether: dichloromethane = 3:1-dichloromethane: methanol = 200:1, V / V) to obtain 480 mg of off-white solid. Yield 35%.
[0078] Synthesis of BA-DAC
[0079]
[0080] BA-NBr (200 mg, 0.45 mmol) was dissolved in 10 mL of ethylene glycol methyl ether, and 600 mg of 1,2-cyclohexanediamine was added thereto. The reaction solution was slowly heated to 110°C and reacted for 12h. Ethylene glycol methyl ether was removed under reduced pressure, and the residue was separated through a silica gel column (dichloromethane:methanol=80:1, V / V) to obtain 90 mg of a yellow s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com