A kind of highly stable free-washing snap-tag probe and its preparation method and application
A high-stability, probe technology, applied in the field of fluorescence imaging, can solve the problems of rhodamine and cyanine dyes that cannot achieve wash-free imaging, difficult to achieve long-term real-time imaging, poor photostability of cyanine dyes, etc. Accuracy, simple and general method, good photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Synthesis of SNAP-tag probe SNAP-DAze.
[0041] Synthesis of intermediate N-(4-hydroxymethyl)benzyl-4-bromo-5-nitro-1,8-naphthalimide (BA-NBr):
[0042]
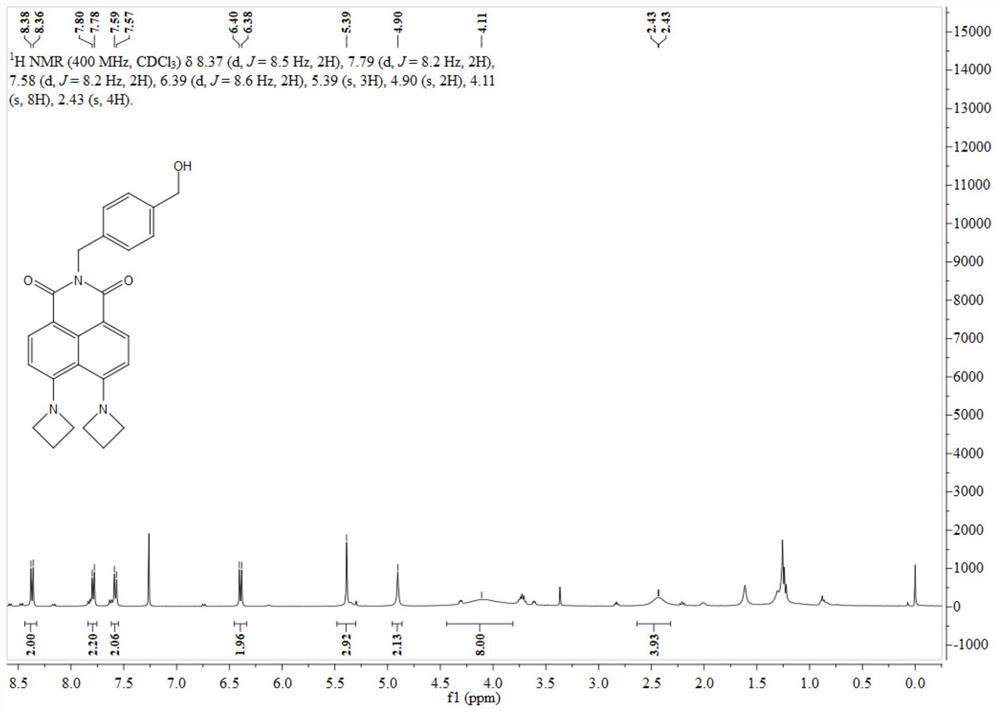
[0043] 4-Bromo-5-nitro-1,8-naphthalimide (1.00 g, 3.11 mmol) was dissolved in 50 mL of ethanol, and 4-aminomethylbenzyl alcohol (853 mg, 6.22 mmol) was added thereto. After 10 h at 80°C, the solvent was distilled off under reduced pressure, and the residue was separated through a silica gel column (dichloromethane:methanol=200:1, V / V) to obtain 480 mg of off-white solid with a yield of 35%. The specific data of its nuclear magnetic spectrum hydrogen spectrum are as follows:
[0044] 1 H NMR (400MHz, DMSO-d 6)δ8.69(d, J=8.1Hz, 2H), 8.50–8.39(m, 2H), 7.35(d, J=8.1Hz, 2H), 7.25(d, J=7.9Hz, 2H), 5.23( s,2H),5.13(t,J=5.8Hz,1H),4.45(d,J=5.5Hz,2H).
[0045] Synthesis of BA-DAze:
[0046]
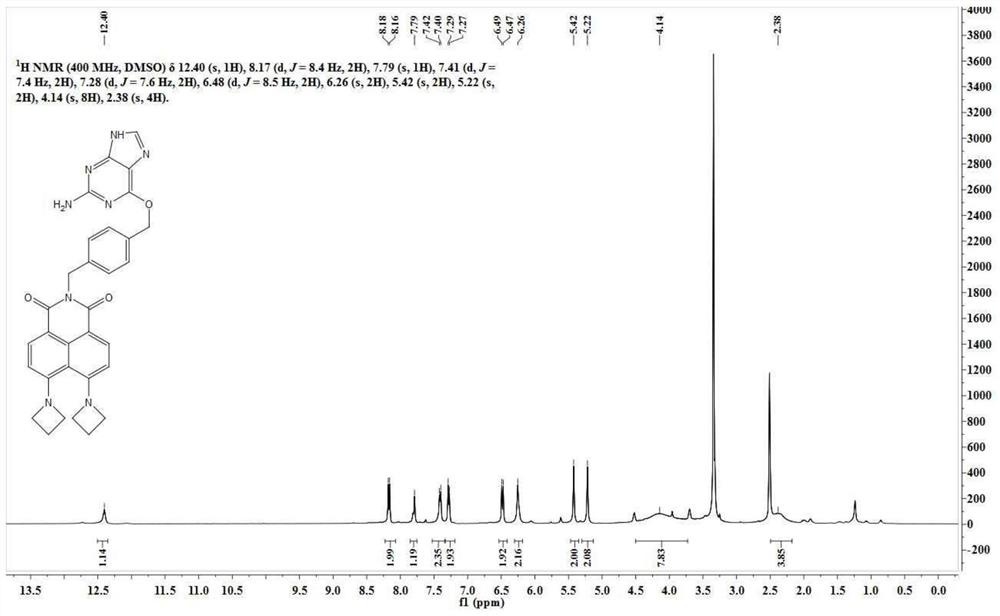
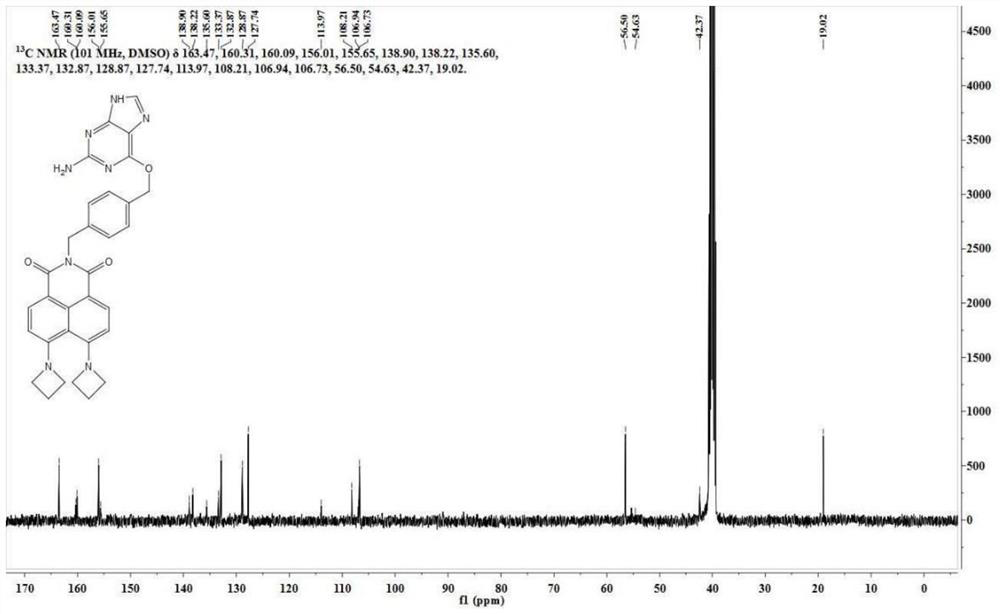
[0047] BA-NBr (300 mg, 0.68 mmol) was dissolved in 30 mL of ethylene glycol methyl ether, and 500 mg of azetidine was added t...
Embodiment 2
[0062] Synthesis of SNAP-tag probe SNAP-DAze.
[0063] Synthesis of intermediate N-(4-hydroxymethyl)benzyl-4-bromo-5-nitro-1,8-naphthalimide (BA-NBr):
[0064]
[0065] 4-Bromo-5-nitro-1,8-naphthalimide (1.50 g, 4.66 mmol) was dissolved in 30 mL of ethanol, and 4-aminomethylbenzyl alcohol (0.75 g, 5.5 mmol) was added thereto. After 9 hours at 40°C, the solvent was distilled off under reduced pressure, and the residue was separated through a silica gel column (dichloromethane:methanol=200:1, V / V) to obtain 656 mg of off-white solid with a yield of 32%.
[0066] Synthesis of BA-DAze:
[0067]
[0068] BA-NBr (420 mg, 0.95 mmol) was dissolved in 21 mL of ethylene glycol methyl ether, and azetidine (420 mg, 7.37 mmol) was added thereto. The reaction solution was slowly heated to 50°C and reacted for 24h. Ethylene glycol methyl ether was removed under reduced pressure, and the residue was separated through a silica gel column (dichloromethane:methanol=60:1, V / V) to obtain ...
Embodiment 3
[0075] Synthesis of SNAP-tag probe SNAP-DAze.
[0076] Synthesis of intermediate N-(4-hydroxymethyl)benzyl-4-bromo-5-nitro-1,8-naphthalimide (BA-NBr):
[0077]
[0078] 4-Bromo-5-nitro-1,8-naphthalimide (1.25 g, 3.88 mmol) was dissolved in 25 mL of ethanol, and 4-aminomethylbenzyl alcohol (5.0 g, 36.5 mmol) was added thereto. After 1 h at 90°C, the solvent was distilled off under reduced pressure, and the residue was separated through a silica gel column (dichloromethane:methanol=200:1, V / V) to obtain 479 mg of off-white solid with a yield of 28%.
[0079] Synthesis of BA-DAze:
[0080]
[0081] BA-NBr (500 mg, 1.13 mmol) was dissolved in 25 mL of ethylene glycol methyl ether, and azetidine (500 mg, 8.77 mmol) was added thereto. The reaction solution was slowly heated to 140°C and reacted for 12h. Ethylene glycol methyl ether was removed under reduced pressure, and the residue was separated through a silica gel column (dichloromethane:methanol=60:1, V / V) to obtain 58 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com