Short-chain dehydrogenase and application
A short-chain dehydrogenase and coenzyme technology, applied in the field of bioengineering, can solve the problem of low yield of ring-opening reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Cloning of Short Chain Dehydrogenase Gene
[0061] Based on the sequence of the gene predicted to be Bacillus subtilis short-chain dehydrogenase (NCBI accession number: WP_013058339) included in NCBI, the PCR primers are designed as follows:
[0062] BsSDR10 f:5ˊ-CGGGATCCGATGAAGTACACAGTTATTACAGGA-3ˊ;
[0063] BsSDR10 r:5ˊ-CCGCTCGAGCATCTCTAGAATCGGATAGATTTGA-3ˊ.
[0064] Among them, the underlined part of the upstream primer is the BamHI restriction site, and the underlined part of the downstream primer is the XhoI restriction site.
[0065] The genomic DNA of Bacillus subtilis CGMCC 1.3358 was used as a template for PCR amplification. The PCR system is as follows:
[0066]
[0067] Amplification procedure: 94°C for 10 minutes, (94°C for 30s, 50°C for 30s, 72°C for 60s) 35 cycles, 72°C for 10 minutes, cooling to 4°C.
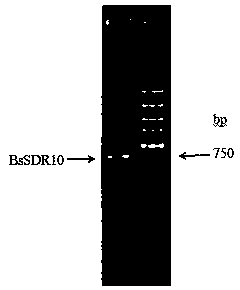
[0068] The PCR product was purified by agarose gel electrophoresis, and the target band of about 750 bp was recovered using the DNA recovery kit ( figure 1 ...
Embodiment 2
[0069] Example 2 Construction of recombinant expression vector plasmid and preparation of recombinant expression transformant
[0070] The short-chain dehydrogenase gene DNA fragment in Example 1 was double-cut with restriction enzymes BamHI and XhoI at 37°C for 12 hours, and the product was purified by agarose gel electrophoresis, and the target fragment was recovered using a DNA recovery kit. Under the action of T4DNA ligase, the target fragment was ligated with the plasmid pET-28b-MBP which was also double digested with restriction enzymes BamHI and XhoI, and ligated overnight at 16°C to obtain a recombinant expression transformant pET-28b -MBP-BsSDR10. Plasmid construction map such as Figure 5 Shown.
[0071] Transform the above-mentioned recombinant expression vector plasmid pET-28b-MBP-BsSDR10 into E. coli BL21 competent cells. The transformation conditions are 42°C, heat shock for 90s, and resistance to kanamycin. Screen the positive recombinants on the plate, pick a sing...
Embodiment 3
[0072] Example 3 Expression of Short Chain Dehydrogenase BsSDR10
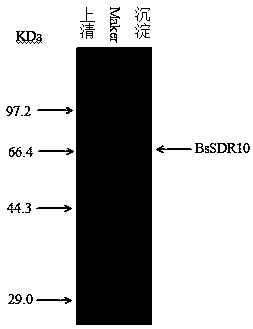
[0073] The recombinant Escherichia coli obtained in Example 2 was inoculated into LB medium (PH 7.0) containing kanamycin, cultured with shaking at 37°C overnight, and inoculated with 100ml LB at 1% (v / v) inoculum The culture medium is placed in a 250ml Erlenmeyer flask, shake cultured on a shaker at 37°C and 180rpm, when the OD of the culture medium 600 When it reached 0.6, IPTG with a final concentration of 0.25 mM was added as an inducer, induced at 20°C for 12 hours, the culture broth was centrifuged, the cells were resuspended in 3 ml of phosphate buffer solution (PH 6.0), and transferred to an EP tube. Ultrasonic disruption under ice bath conditions, low-temperature (4°C) centrifugation to collect the supernatant, which is the crude enzyme solution of recombinant short-chain dehydrogenase. The crude enzyme solution and the precipitate are analyzed by polyacrylamide gel electrophoresis, such as image 3 Shown...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap