Methods for treating cancer with anti-PD-1 antibodies
A PD-1, cancer technology, applied in the direction of antibody medical components, antibodies, chemical instruments and methods, can solve problems such as impaired immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
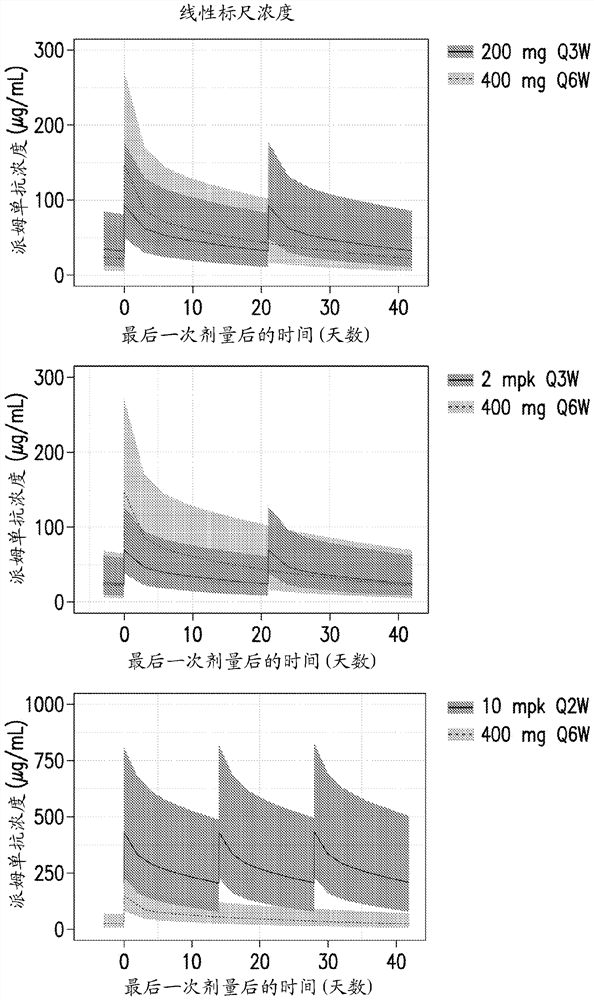
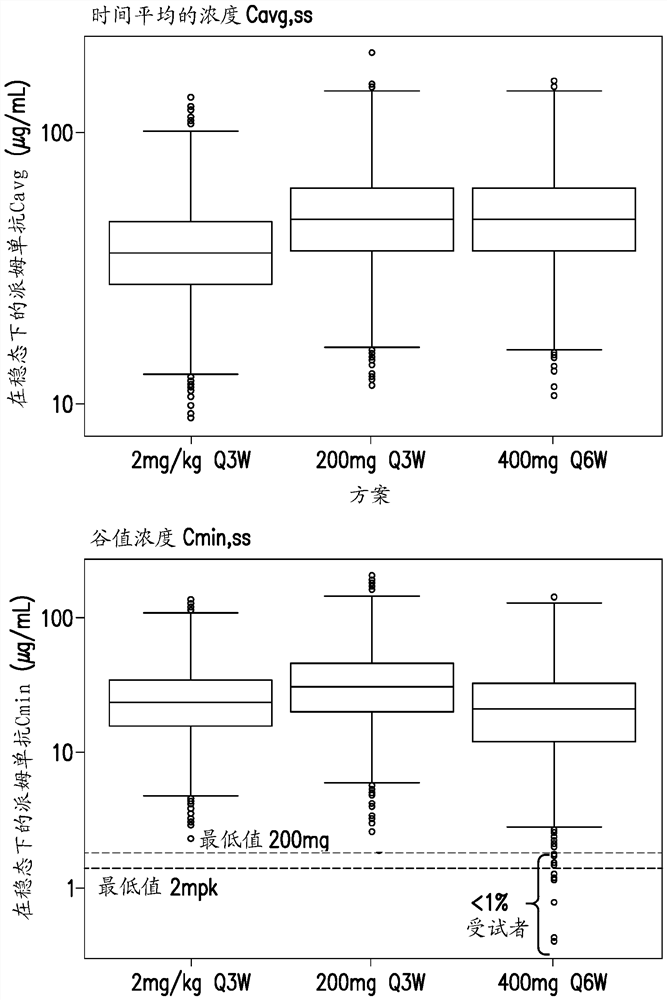
[0262] Six-times-weekly (Q6W) dosing schedule for pembrolizumab across multiple tumor types based on assessments using modeling and simulation
[0263] Pembrolizumab, an anti-PD-1 checkpoint inhibitor currently approved for use in multiple cancer indications, has demonstrated safety and efficacy when administered at doses of 200 mg or 2 mg / kg Q3W. Alternative extended dosing regimens would provide convenience and flexibility benefits to both patients and prescribers. Robust characterization of pembrolizumab pharmacokinetics (PK) and exposure (concentration)-response (E-R) relationships for both efficacy and safety allows model-based approaches to support alternative dosing regimens for pembrolizumab .
[0264] Dosing for the Q6W schedule of pembrolizumab was selected by matching exposure to approved Q3W (200 mg and 2 mg / kg) regimens after PK steady state was achieved; based on knowledge of the E-R, the efficacy between regimens was Build bridges with security. Using an esta...
Embodiment 2
[0270] A phase 1 randomized clinical study of pembrolizumab evaluating the safety and tolerability of intravenous infusion of 400 mg pembrolizumab Q6W in participants with advanced melanoma
[0271]The study was designed to evaluate the pharmacokinetics (PK), safety and tolerability of pembrolizumab when administered every 6 weeks (Q6W). Cohorts of 100 participants were given 400 mg of pembrolizumab Q6W. PK, potency and safety data are collected from this participant cohort. Male / female participants at least 18 years of age with advanced melanoma were enrolled in the study. Stratification based on age, sex, or other characteristics was not used in this study.
[0272] Participants received an IV infusion of 400 mg pembrolizumab Q6W from cycles 1 to 18. PK, potency and safety data were collected from these participants. The results provide preliminary PK, efficacy and safety data for pembrolizumab when administered Q6W. Based on a robust understanding of the clinical pharm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com