Use of apof protein in the preparation or screening of diagnostic products for schizophrenia
A schizophrenia and protein technology, applied in the direction of disease diagnosis, medical automation diagnosis, analysis of materials, etc., can solve the problem of undiagnosed schizophrenia and other problems, and achieve the effect of high diagnostic efficiency and stable performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
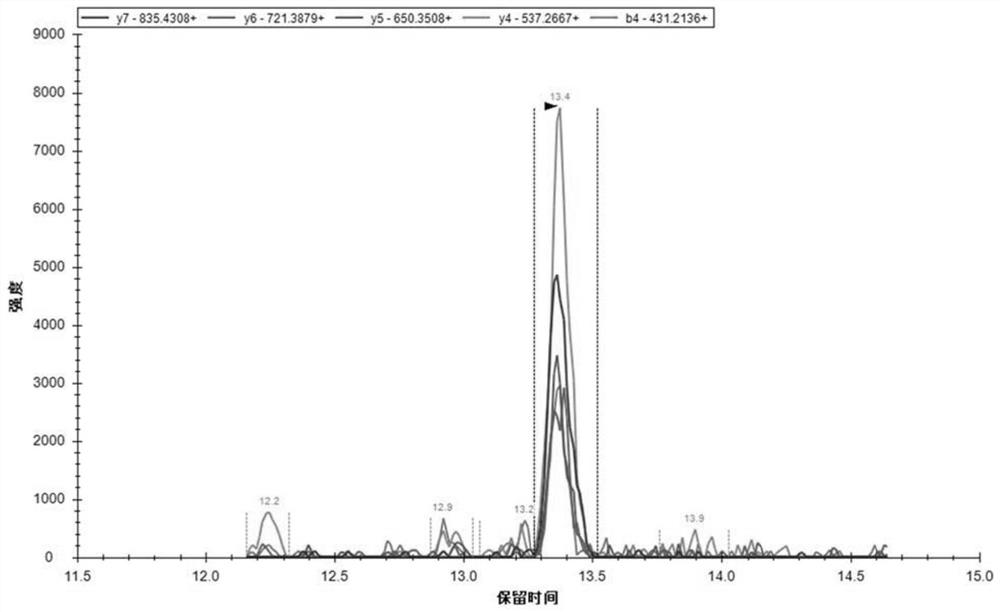
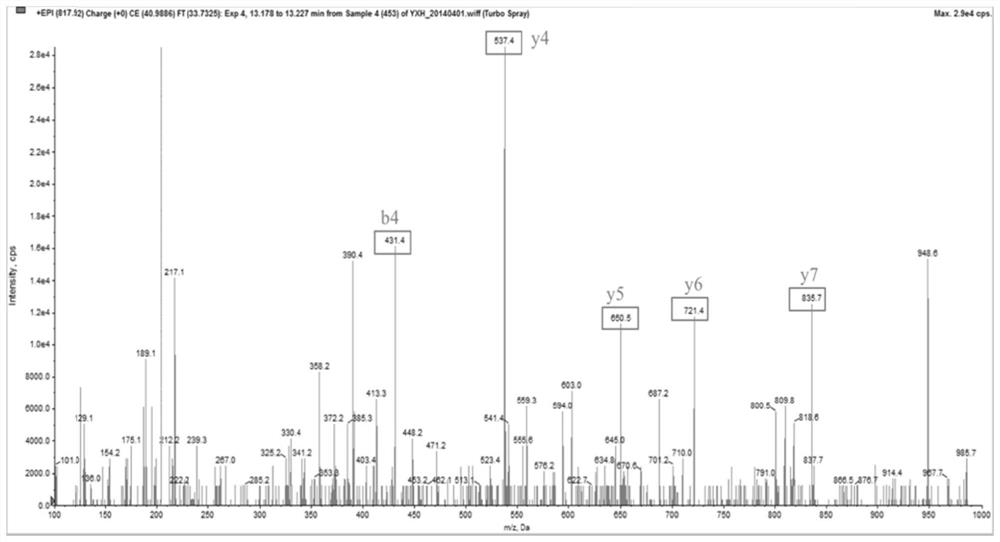
[0120] 1. Sample Collection
[0121] 110 patients with schizophrenia diagnosed by ICD-11 and 109 normal controls were screened and collected from the Fourth People's Hospital of Wuhu City, Anhui Province. The patients were randomly divided into a discovery set (60 cases) and a validation set (50 cases), and then 61 cases matched with age, gender, and BMI were selected from the normal controls to enter the discovery set, and the remaining 48 cases entered the validation set, that is, the discovery set had a total of 61 cases. There are 60 patients and 61 healthy people, and the validation set contains 50 patients and 48 healthy people. All the enrolled individuals had no physical diseases such as diabetes, malignant tumor, fever, inflammation, etc., and all female individuals were not pregnant. This study has been reviewed by the Ethics Committee of the Fourth People's Hospital of Wuhu City, and the experimental protocol is in line with ethical principles, and all participants...
Embodiment 2
[0178] Example 2 Construction of a diagnostic model
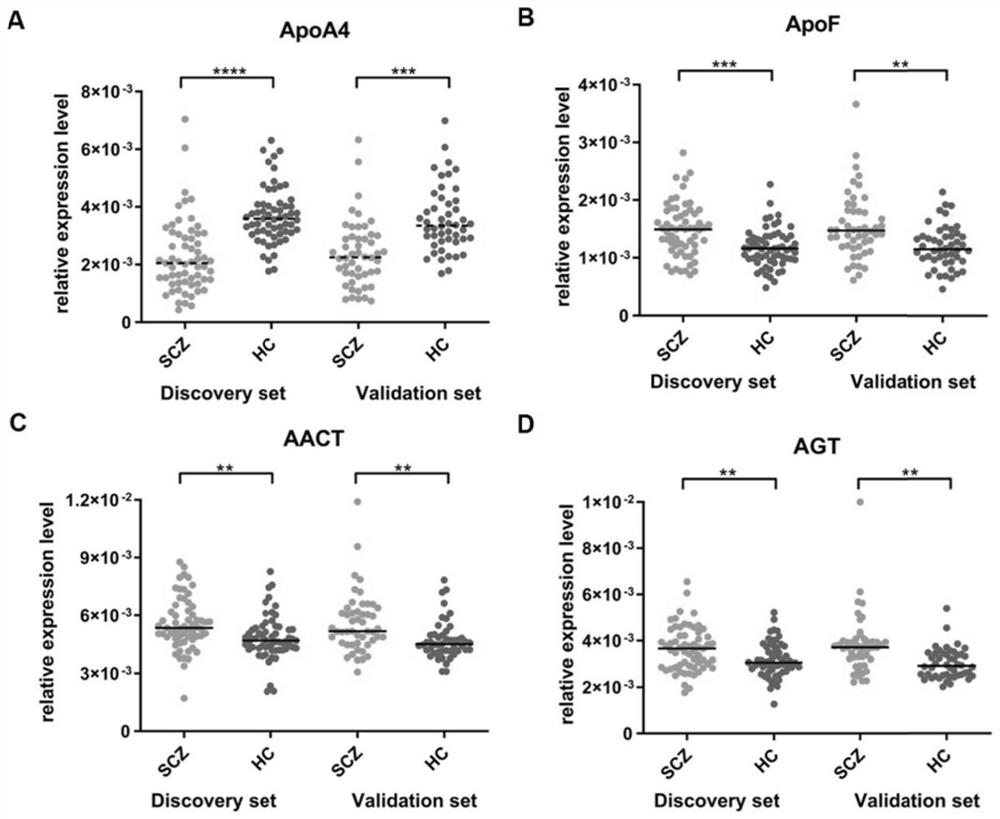
[0179] In the process of constructing the diagnostic model, each discovery set sample in Example 1 is used as a training group to construct a diagnostic model; each verification set sample is used to test the diagnostic efficiency of the model. First, the AUC of each single protein in the discovery set and the validation set in the serum was detected, and the results are shown in Table 4.
[0180] Table 4 AUC of individual proteins
[0181] protein Discovery Set AUC Validation set AUC ApoA4 0.840 0.791 ApoF 0.709 0.708 AGT 0.646 0.763 ACT 0.676 0.710
[0182] Then, age, gender, BMI and various protein variables were arranged and combined to build a logistic regression model in turn. The ROC curve of each group is as follows Figures 4-1 to 4-7 , calculate the AIC and AUC of each model, and the results are shown in Table 3.
[0183] Table 5 Serum Apolipoprotein Combination Con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com