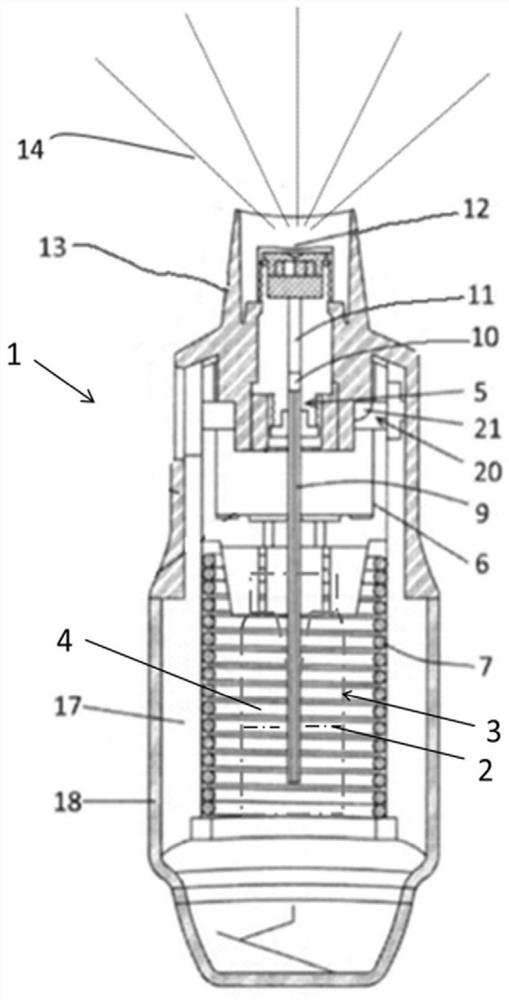
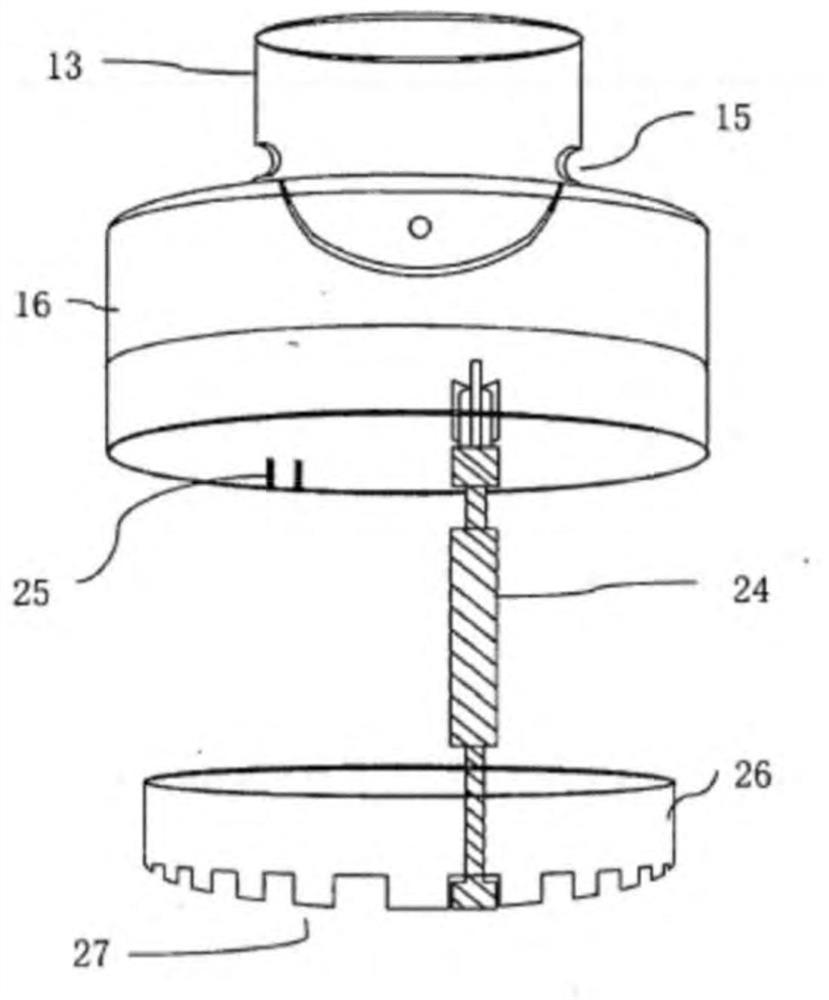
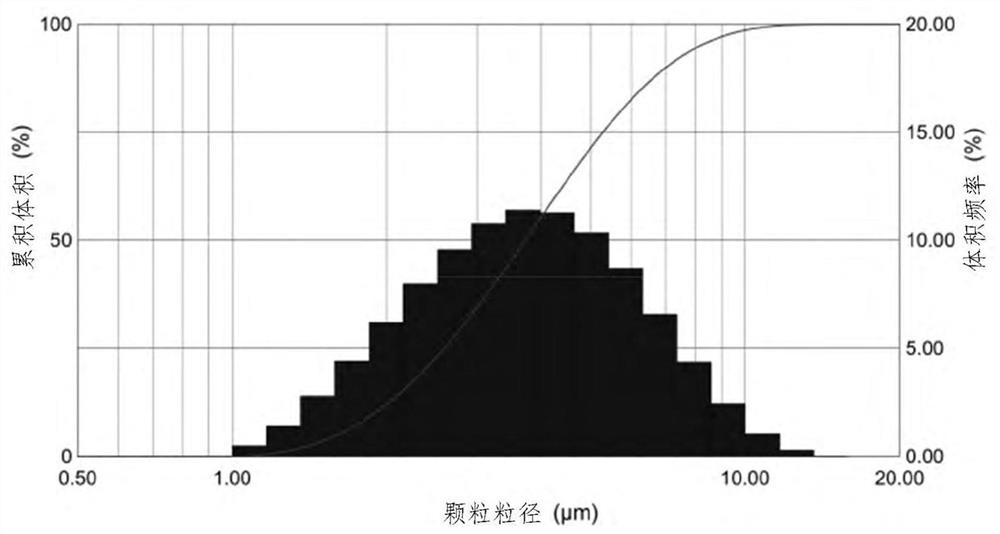
Inhalable formulation of a solution containing formoterol fumarate and aclidinium bromide
A solvent and inhaler technology, applied in the field of salt or solvate, treatment of asthma and chronic obstructive pulmonary disease, aclidinium bromide and formoterol fumarate, can solve problems such as difficulty in administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Synthesis of aclidinium bromide:
[0082] To acetonitrile (100 mL) was added (R)-quinuclidin-3-yl-2-hydroxy-2,2-bis(thiophen-2-yl)acetate (10 g, 28.7 mmol) and (3-bromopropane oxy)benzene (12.3 g, 57.4 mmol). The reaction mixture was heated to 80-90°C and stirred for 8 hours before a white solid formed. The mixture was cooled to 20-25°C, filtered and washed with ice-cold acetonitrile (10 mL), repeated filtration and washing three times, and dried under vacuum at 50°C to obtain a white solid (15.4 g, 27.4 mmol). The yield of aclidinium bromide was 95%, and the HPLC purity was 99.8%.
Embodiment 2
[0084] Inhalation solutions of Sample 1, Sample 2, and Sample 3 were prepared with different amounts of disodium EDTA dihydrate:
[0085] The ingredients are listed in Table 1. According to Table 1, 50% benzalkonium chloride was dissolved in purified water three times, and then transferred to a 100ml volumetric flask. Add disodium edetate dihydrate and anhydrous citric acid to the solution according to Table 1, and sonicate until completely dissolved; then, add formoterol fumarate and aclidinium bromide to the solution according to Table 1 and sonicated until completely dissolved. Disodium EDTA dihydrate was added to the solution according to Table 1, and then sonicated until completely dissolved. Finally, the volumetric flask was adjusted to volume with purified water, and the pH value was adjusted to 3.0 with 1N HCl. Sample 1, Sample 2 and Sample 3 solutions remained substantially clear. The results are shown in Table 2.
[0086] Table 1. Ingredient content of inhalatio...
Embodiment 3
[0091] Preparation of sample 4, sample 5, sample 6, sample 7 and sample 8 different pH value inhalation solution:
[0092] The ingredients are listed in Table 3. According to Table 3, 50% benzalkonium chloride was dissolved in purified water three times, and then transferred to a 100ml volumetric flask. Add disodium EDTA dihydrate and anhydrous citric acid to the solution according to Table 3, and sonicate until completely dissolved; then, add formoterol fumarate and aclidinium bromide to the solution according to Table 3 and sonicated until completely dissolved. Finally, the volumetric flask was made to volume with pure water, and the pH value was adjusted to the target value with 1N HCl. Samples 4-8 solutions remained essentially clear. The results are shown in Table 4.
[0093] Table 3. Ingredient content of inhalation preparation samples 4-8
[0094] components Sample 4 Sample 5 Sample 6 Sample 7 Sample 8 Aclidinium bromide 20mg 20mg 20m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com