Lta4h inhibitors for the treatment of hidradenitis suppurativa
A technology of hidradenitis suppurativa and inhibitors, applied in the direction of medical preparations containing active ingredients, pill delivery, organic active ingredients, etc., which can solve problems such as debility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0099]Embodiment 2A relates to the method of embodiment 2 comprising administering to the subject a therapeutically effective amount of a compound of formula (II) or a pharmaceutically acceptable salt thereof,
[0100]
[0101] wherein the variables R1, R2 and Y have the meanings as defined in Example 1.
[0102] Embodiment 2B relates to the method of embodiment 2 comprising administering to the subject a therapeutically effective amount of a compound of formula (III) or a pharmaceutically acceptable salt thereof,
[0103]
[0104] wherein the variables R1, R2 and Y have the meanings as defined in Example 1.
[0105] Embodiment 2C is directed to the method of embodiment 2, comprising administering to the subject a therapeutically effective amount of a compound of formula (IV) or a pharmaceutically acceptable salt thereof,
[0106]
[0107] wherein the variables R1, R2 and Y have the meanings as defined in Example 1.
[0108] Embodiment 2D is directed to the method o...
example 1
[0368] Example 1: (S)-3-amino-4-(5-(4-((5-chloro-3-fluoropyridin-2-yl)oxy)phenyl)-2H-tetrazol-2-yl) Butyric Acid (Form B)
[0369]
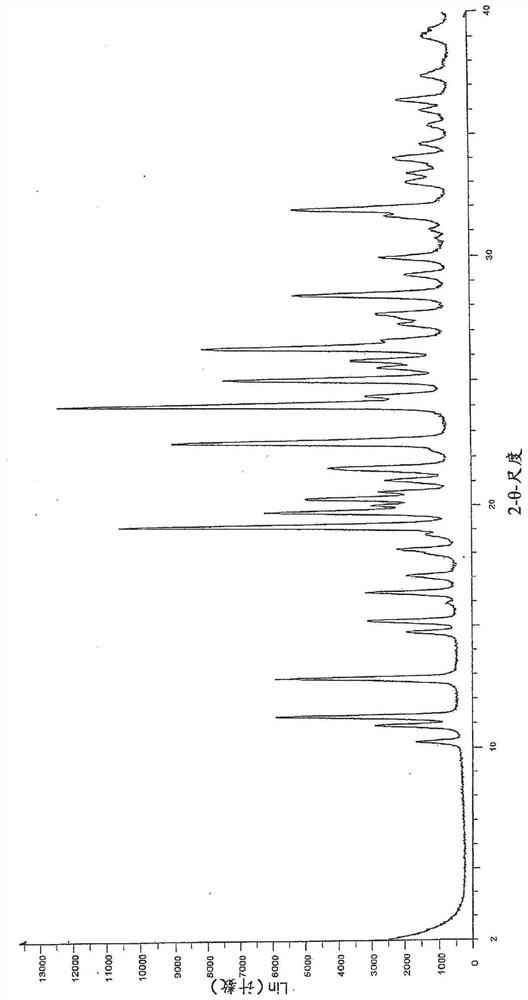
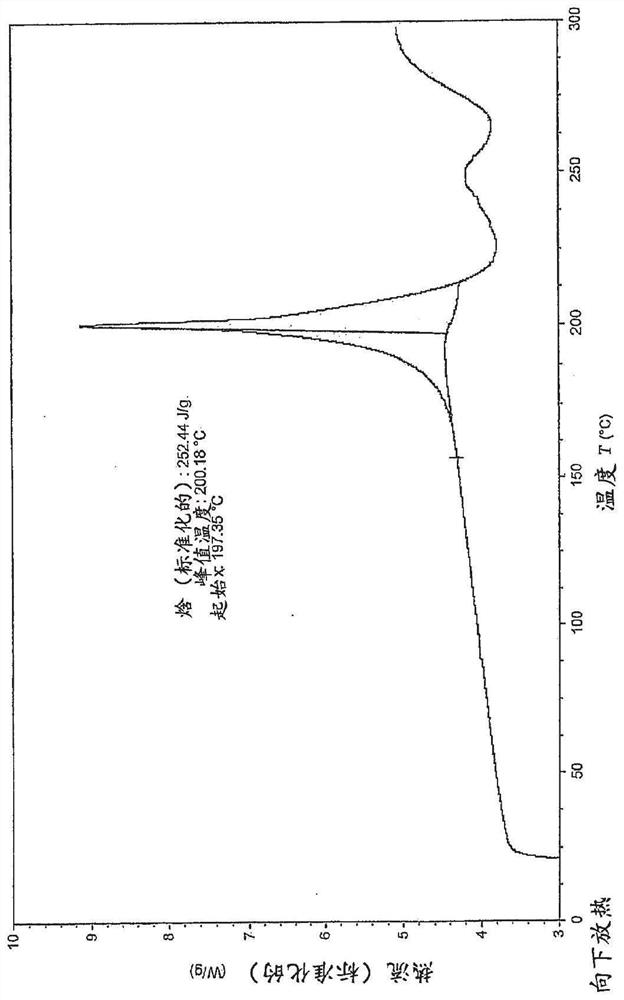
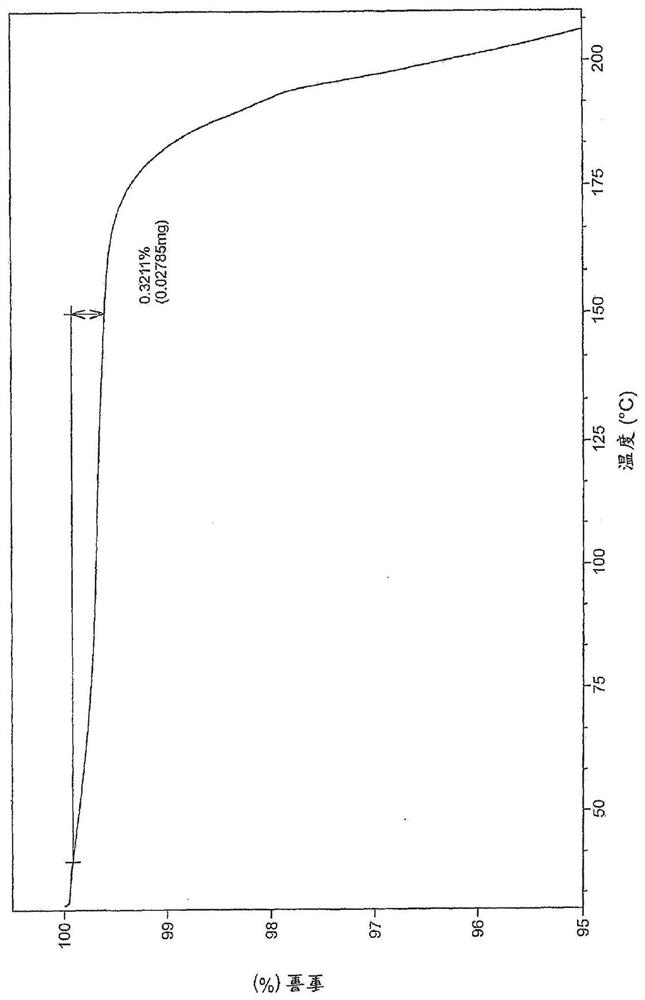
[0370] Example 29 (28 g, 35 mmol) as described in WO 2015 / 092740 and a solvent mixture containing 360 g water and 40 g THF were mixed together and stirred for 20 minutes. The mixture was filtered and washed with aqueous NaHCO 3 The filtrate was adjusted to pH=5. Stirring was continued for 18 h before the mixture was filtered to give 25.6 g of the free acid (Int-1 ) in a wet cake which was used without further purification to prepare polymorph Form B. 505 mg of free acid (Int-1) was weighed into a 20 ml glass vial and 6 mL of methanol was added. The slurry was heated to 50 °C and stirred using a magnetic stirrer for 4 days. The suspension was cooled to room temperature and filtered. The recovered solid was dried under vacuum at 40 °C for 2.5 h. The white solid was analyzed by XRPD, DSC and TGA (respectively Figure 1-3 ).
[0371] An al...
example 2
[0386] Compound of Example 1 as a formulation of capsules
[0387]
[0388] 1 of vegetable origin
[0389] 2 removed during processing
[0390] 3 The composition of the capsule shell is given in the table below
[0391] Components of a capsule shell
[0392]
[0393] E: Refers to the official number used by the European Union for colorants
[0394] Regulation (EU) 231 / 2012: Commission Regulation (EU) laying down the specifications for food additives. Capsules were purchased from Lonza (now Capsugel)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com