Application of deferoxamine mesylate in preparation of antitumor drugs
A technology of deferoxamine mesylate and anti-tumor drug, applied in the field of anti-tumor drugs, can solve the problem of no report of deferoxamine mesylate, and achieve the effects of reducing iron load, inhibiting growth, and inhibiting HCC proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
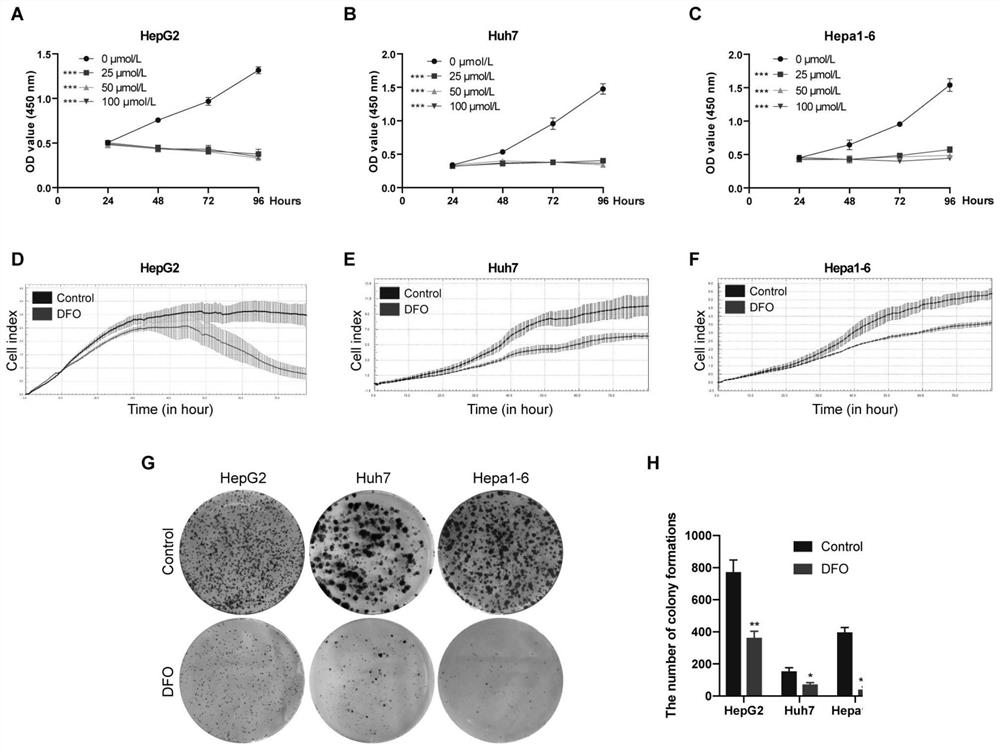
[0032] Example 1 CCK-8 method to detect the effect of DFO on the proliferation of liver cancer cells
[0033] HepG2, Huh7 and Hepa1-6 cells were treated with different concentrations of DFO (0, 25, 50, 100mol / L). cells in 3×10 per well 3 Cells were seeded in 96-well plates. Some cells were incubated with different concentrations of DFO (25, 50, 100 mol / L, DFO was purchased from Novartis Pharmaceutical Co., Ltd.), and other cells were used as the control group. After culturing for 12 hours, 10 mol / L of CCK-8 (Dojindo, Kumamoto Island, Japan) cells were added to each well at designated time points (24, 48, 72 and 96 hours). After the cells were incubated for 2 hours, the optical density was measured at 450 nm with a microplate reader.
[0034]The result is as figure 1 As shown in A-C, the cell proliferation of 25, 50, 100mol / L DFO groups was significantly different from that of the control group. Therefore, we chose DFO with a dose of 25mol / L for 48h further experiment.
Embodiment 2
[0035] Example 2 RTCA real-time cell analysis system dynamically monitors the effect of DFO on the proliferation of liver cancer cells
[0036] The growth rate of HCC cells was measured by RTCA real-time label-free cell function analyzer (IXL8, ACEA, USA). Place the RTCA real-time label-free cell function analyzer at 37°C, 5% CO 2 In a humidified incubator, add 100ul / well medium containing 10% FBS to an 8-well electronic plate, and immediately record the background reading. Then 48,000 liver cancer cells were added to 300 rat liver cell suspensions. After incubation for 24 hours, DFO was added to the medium (the final concentration of DFO was 25 mol / L), and the cell growth rate was recorded every 1 hour for continuous monitoring for 96 hours.
[0037] The results showed that 25mol / L DFO treatment significantly inhibited the proliferation of HepG2, Huh7 and Hepa1-6 cells ( figure 1 D-F).
Embodiment 3
[0038] Example 3 Clonogenic test
[0039] HepG2, Huh7 and Hepa1-6 cells were seeded in 6-well plates (500 cells / well), and the experimental group was treated with 25mol / L DFO. Cells were incubated at 37°C for 10 days. On the 10th day, the cells were washed 3 times with PBS. Fix with 4% paraformaldehyde for 30 min, stain with 0.5% crystal violet for 30 min at room temperature, observe and count cell colonies.
[0040] The results of the colony formation experiment showed that compared with the control group, the proliferation ability of the cells treated with 25mol / L DFO decreased significantly. Compared with the control group, the colony formation rates of HepG2, Huh7 and Hepa1-6 cells decreased by 52.74%, 52.13% and 89.07% after DFO treatment (Pfigure 1 G, H).
[0041] From the test results of Examples 1-3, it can be seen that DFO can inhibit the proliferation of liver cancer cells in vitro.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap