Hip arthroplasty trial systems and associated medical devices, methods, and kits
A hip joint replacement and test system technology, applied in the field of medical devices, can solve the problems of repeated hip joint dislocation, time-consuming and complicated problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0144] Various embodiments of the hip joint replacement test system, the medical equipment used in hip joint replacement, the method of using the hip joint replacement test system and the complete set of devices are described and shown in detail below and in the accompanying drawings. The description and illustrations of these examples are intended to enable those skilled in the art to make and use hip replacement trial systems, medical devices, kits containing hip replacement trial systems, and to practice hip replacement trial systems and / or The method of using the medical device is not intended to limit the scope of the claims in any way.
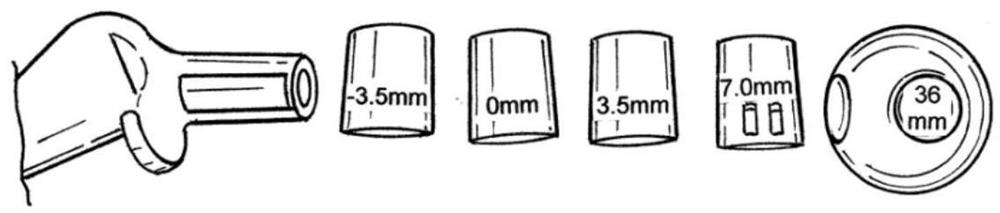
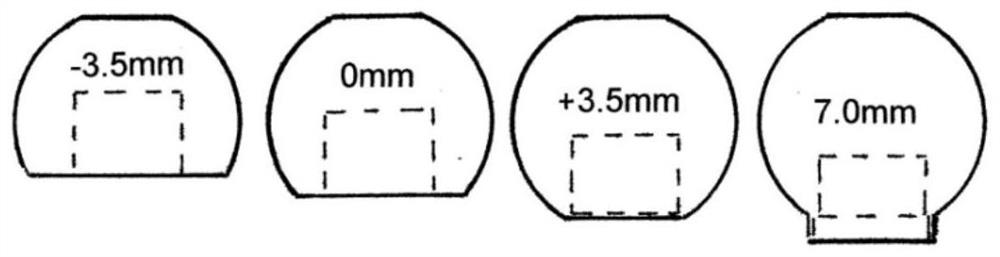
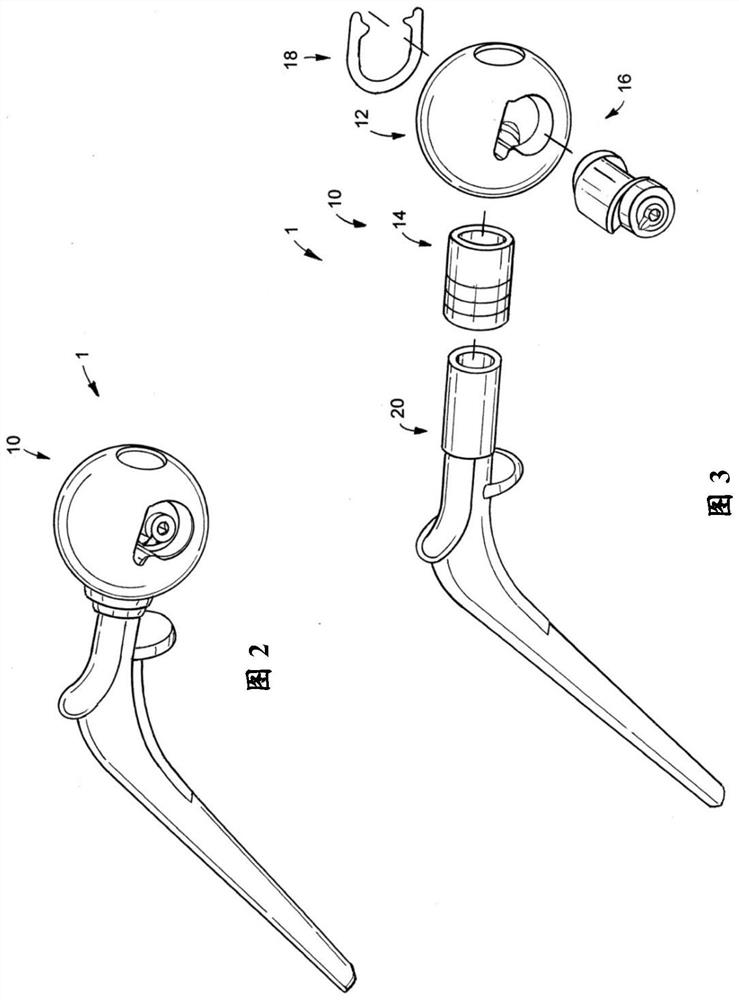
[0145] Figure 2 to Figure 38 Shown is a first exemplary hip replacement trial system 1 comprising a medical instrument 10 and a femoral stem 20 . The medical device 10 has a head member 12 , a spacer 14 , a shaft 16 and a lock 18 . exist figure 2 In some of the drawings, the medical device 10 is shown detachably attached to the femora...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com