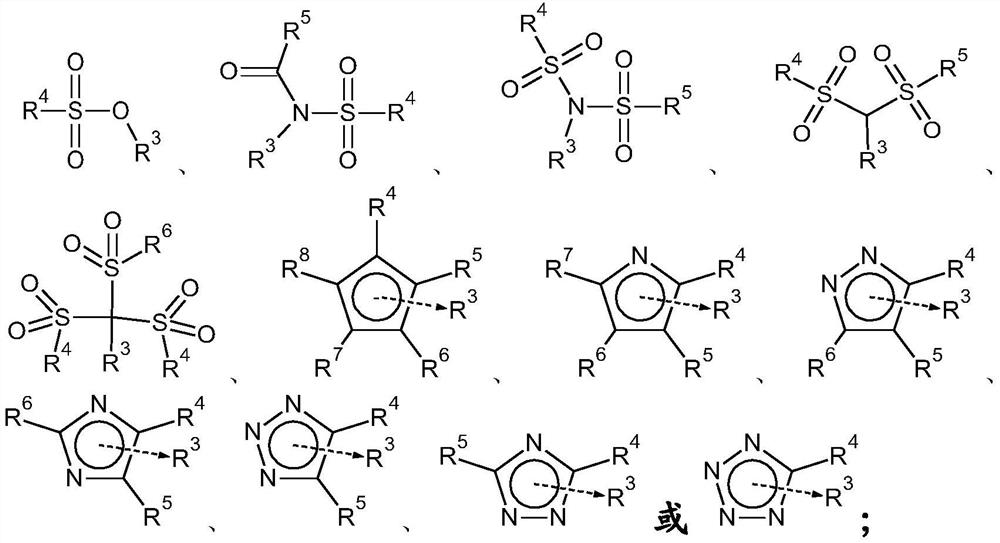
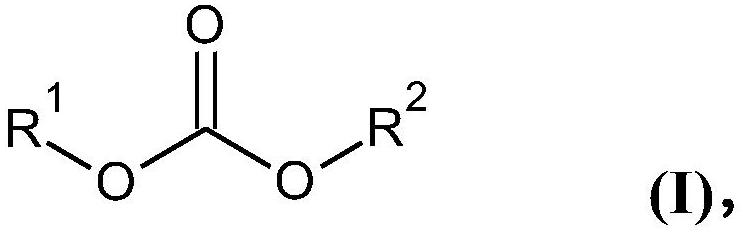
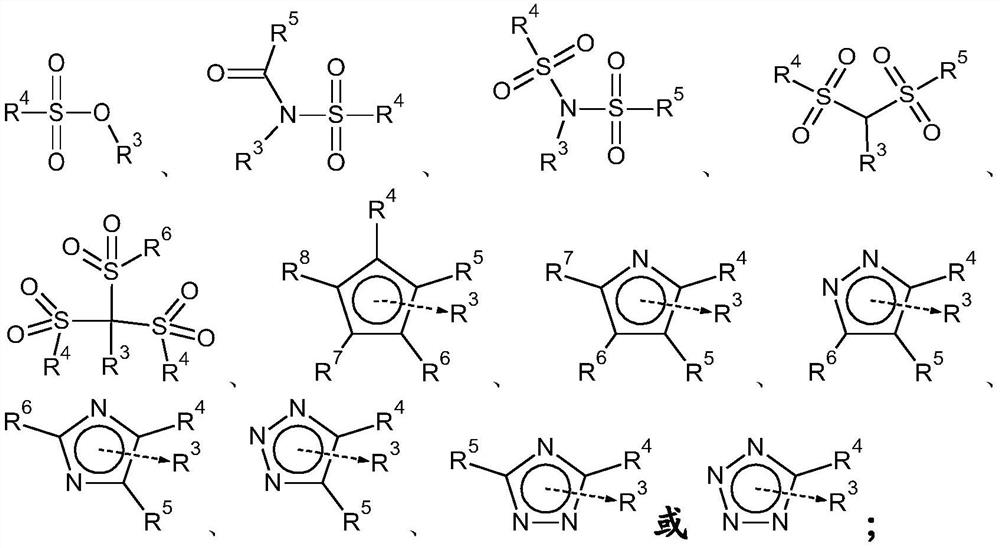
Carbonate solvents for non-aqueous electrolytes for metal and metal-ion batteries
A metal ion, battery pack technology, applied in non-aqueous electrolytes, hybrid capacitor electrolytes, electrolytes, etc., can solve problems that do not represent the best solution for the problem of aluminum dissolution in anodes, and do not represent the best substitute for conventional solvents.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0304] The preparation of carbonate solvents of the present invention on a small scale is most conveniently accomplished by base-catalyzed transesterification of readily available dimethyl carbonate, diethyl carbonate, ethylene carbonate or propylene with aliphatic alcohols in the presence of a suitable catalyst. The transesterification of carbonates follows the same rules as transesterification of other esters and is a typical equilibrium reaction that can be easily controlled using Le Chatelier's principle. The ratio between alcohol and carbonate determines the ratio of products in a perfectly balanced reaction mixture. If complete substitution is desired, excess alcohol should be used. If the desired product is a mixed carbonate, the molar ratio should be close to 1, or a slight excess of the starting carbonate should be used. During the course of the reaction, it is desirable to steadily remove the reaction product from the reaction mixture; this allows the reaction to pr...
Embodiment 60
[0337] Example 60 involves measuring the temperature ranges of the electrolytes of the present invention and conventional electrolytes by performing digital scanning calorimetry (DSC) experiments.
Embodiment 61
[0338] Example 61 involved measuring the discharge capacity versus cycle number of three cells; two cells containing the electrolyte of the invention and one containing a conventional electrolyte.
[0339] The preparation of carbonate solvent of the present invention
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com