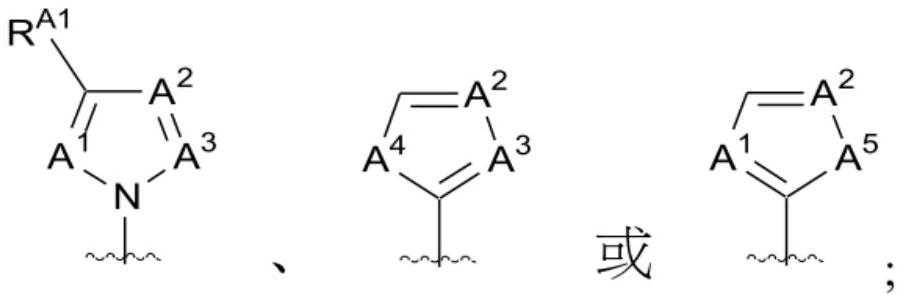
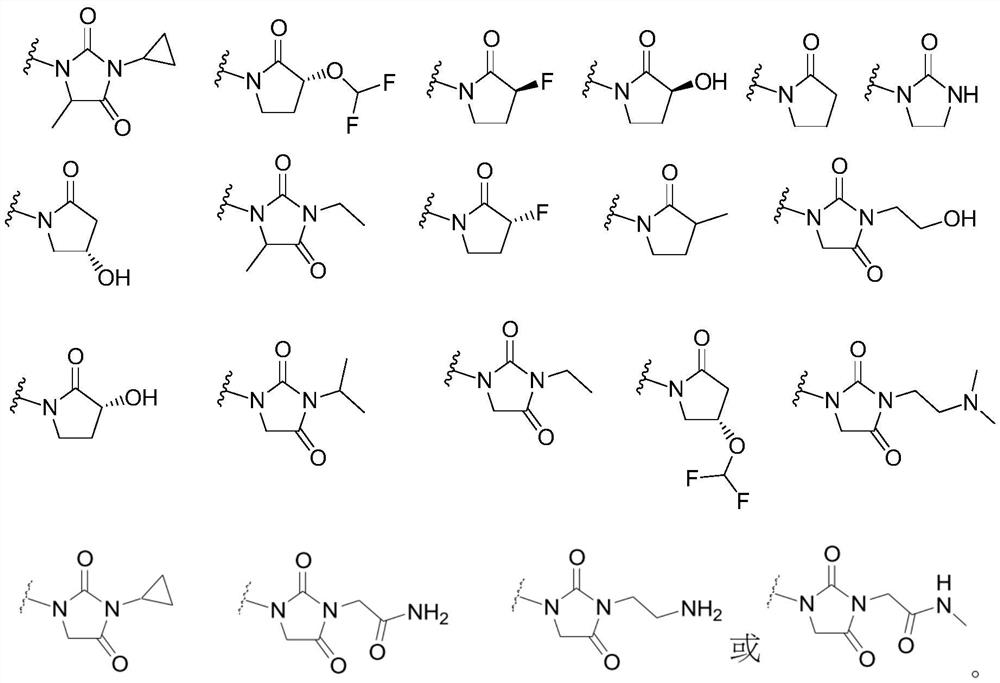
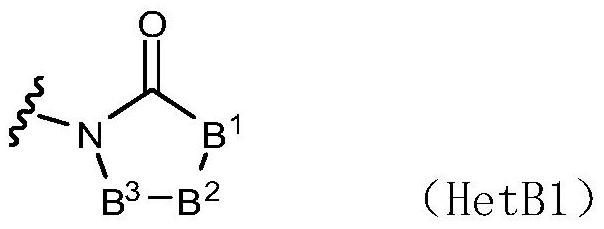1-((s)-1-(3-chloro-5-fluoro-2-((4-(1h-pyrazol-1-yl)-2-methylquinolin-8-yloxy)methyl)phenyl)ethyl)-imidazolidine-2,4-dione derivatives and related compounds as bradykinin (BK) b2 receptor antagonist for treating skin diseases
A compound, C1-C3 technology, applied in skin diseases, drug combinations, diseases, etc., can solve the problems of low bioavailability, hindering practicality, low metabolic stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0178] Example 1: Preparation of Compound No. 1
[0179]
[0180] (S)-2-(3-(1-(3-chloro-5-fluoro-2-((4-(4-fluoro-1H-pyrazol-1-yl)-2-methylquinoline-8 -yloxy)methyl)phenyl)ethyl)-2,5-two-side oxyimidazolidin-1-yl)acetamide
[0181] step a. Synthesis of 3-amino-5-fluoro-2-methylbenzoic acid methyl ester
[0182] Methyl 5-fluoro-2-methyl-3-nitrobenzoate [Gillmore, A.T. et al., Org. Process Res. Dev. 2012, 16, 1897-1904] (4.69 g; 22 mmol) was dissolved in MeOH (100 mL ) and added palladium-10% Pd on charcoal (200 mg). The solution was flushed with nitrogen and vacuumed three times, then flushed with hydrogen. The reaction mixture was stirred vigorously under 1 atm of hydrogen. After completion of the reaction as indicated by TLC (21 h), the solution was filtered through silica gel. The filter cake was washed with methanol (5 x 20 mL). The filtrate was concentrated under reduced pressure, and the residue was purified by flash chromatography on silica gel (eluting with EA...
example 2
[0213] Example 2: Preparation of Compound No. 2
[0214]
[0215] 1-((S)-1-(3-chloro-5-fluoro-2-((4-(4-fluoro-1H-pyrazol-1-yl)-2-methylquinolin-8-yloxy Base) methyl) phenyl) ethyl) -3-ethyl-5-methylimidazolidine-2,4-dione diastereomer B
[0216] step a. Diastereomer of methyl 2-((S)-1-(3-chloro-5-fluoro-2-((4-methoxyphenoxy)methyl)phenyl)ethylamino)propanoate Synthesis of A and B
[0217] (S)-1-(3-Chloro-5-fluoro-2-(4-methoxyphenoxy)methyl)phenyl)ethylamine (220 mg, 710 μmol), methyl pyruvate (72.0 μL, 79.8 mg, 781 μmol) and borane-pyridine-complex (107.6 μl, 1.06 mmol) were dissolved in propan-2-ol (5 mL). About 10 μL of 0.5M aqueous HCl was added and the reaction mixture was stirred overnight at RT. After the reaction was complete (TLC), the solution was poured onto an SCX-cartridge (Phenomenex, StrataSCX, 0.6 mmol / g) and eluted with methanol. The resin was then washed with about 8M methanolic ammonia solution to give the crude product after evaporation of the solv...
example 3
[0230] Example 3: Preparation of Compound No. 3
[0231]
[0232] (S)-1-(1-(3-chloro-5-fluoro-2-((4-(4-fluoro-1H-pyrazol-1-yl)-2-methylquinolin-8-yloxy Base) methyl) phenyl) ethyl) imidazolidin-2-one
[0233] step a. Synthesis of (S)-tert-butyl 2-(1-(3-chloro-5-fluoro-2-((4-methoxyphenoxy)methyl)phenyl)ethylamino)ethylcarbamate
[0234] (S)-1-(3-chloro-5-fluoro-2-(4-methoxyphenoxy)methyl)phenyl)ethylamine (100mg, 323μmol), N-Boc-2-aminoethyl Aldehyde (113.1 μl, 355 μmol) and borane-pyridine-complex (48.9 μl, 484 μmol) were dissolved in 2-propanol (4 mL). About 10 μL of 0.5M HCl solution was added and the reaction mixture was stirred overnight at RT. After the reaction was complete (TLC), saturated NaHCO was added 3 aqueous solution and the aqueous phase was extracted with DCM (3x). Combined organic layers were passed through Na 2 SO 4 Dry and evaporate to dryness under reduced pressure after filtration. The crude product was purified by flash chromatography on sil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com