UBE3A for treating angel syndrome
A protein and polynucleotide technology, applied in the field of UBE3A for the treatment of Angelman syndrome
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
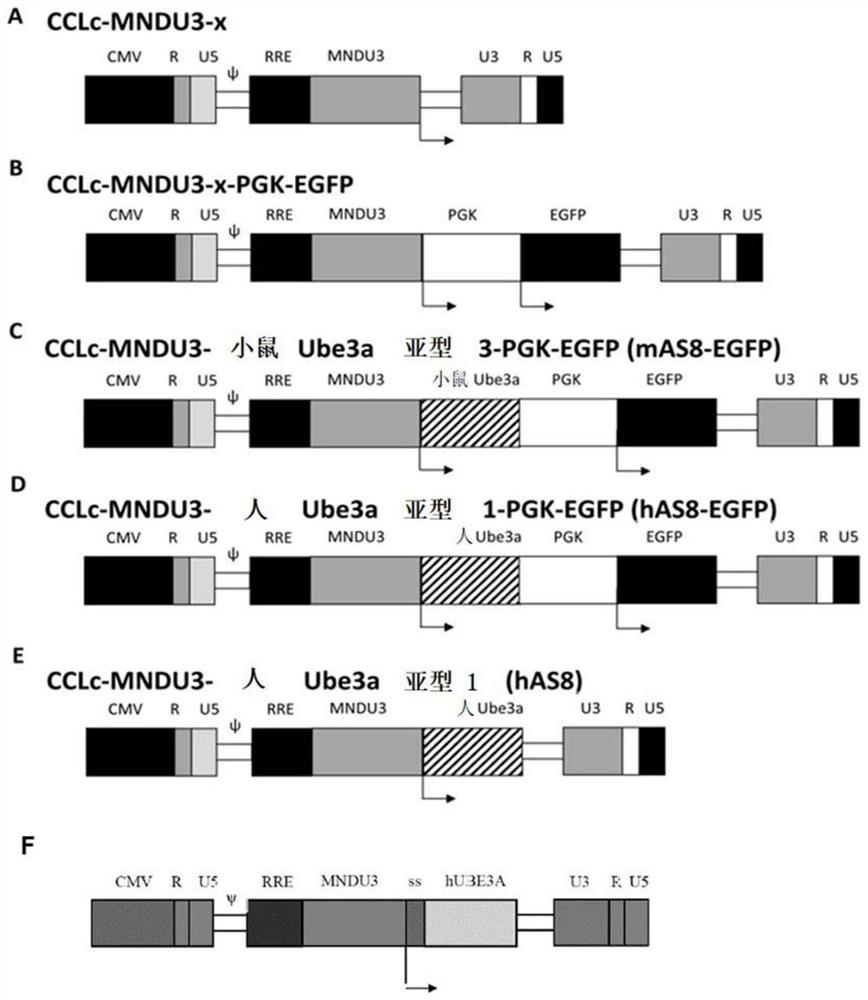
[0140] Polynucleotides and Peptides
[0141] The present disclosure provides polynucleotides encoding ubiquitin protein ligase E3A (Ube3a) proteins, polypeptides or biological equivalents thereof. In some embodiments, polynucleotides are recombinant and / or isolated. In some embodiments, the Ube3a protein, polypeptide, or biological equivalent thereof comprises one or more glycosylation sites.
[0142] Also provided are Ube3a proteins, polypeptides or biological equivalents thereof comprising one or more glycosylation sites. In some embodiments, the Ube3a protein, polypeptide or biological equivalent thereof is isolated, engineered and / or recombinant.
[0143] In some embodiments, the Ube3a protein, polypeptide or biological equivalent thereof is not a wild-type Ube3a protein, for example comprising a sequence selected from SEQ ID NO: 8, 10, 12, 20, 22 or 24 or any natural variant thereof . Such Ube3a proteins, polypeptides or biological equivalents thereof are also referre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com