Methods of using and compositions comprising dopamine reuptake inhibitors
A technology for solvates, pharmaceuticals, in the field of racemic and optically pure metabolites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13-14
[0158] Examples 13-14 describe methods for determining the binding affinity of compounds of the invention and the binding affinities determined using these methods.
[0159] Finally, Example 15 describes oral formulations comprising compounds of the invention.
Embodiment 1
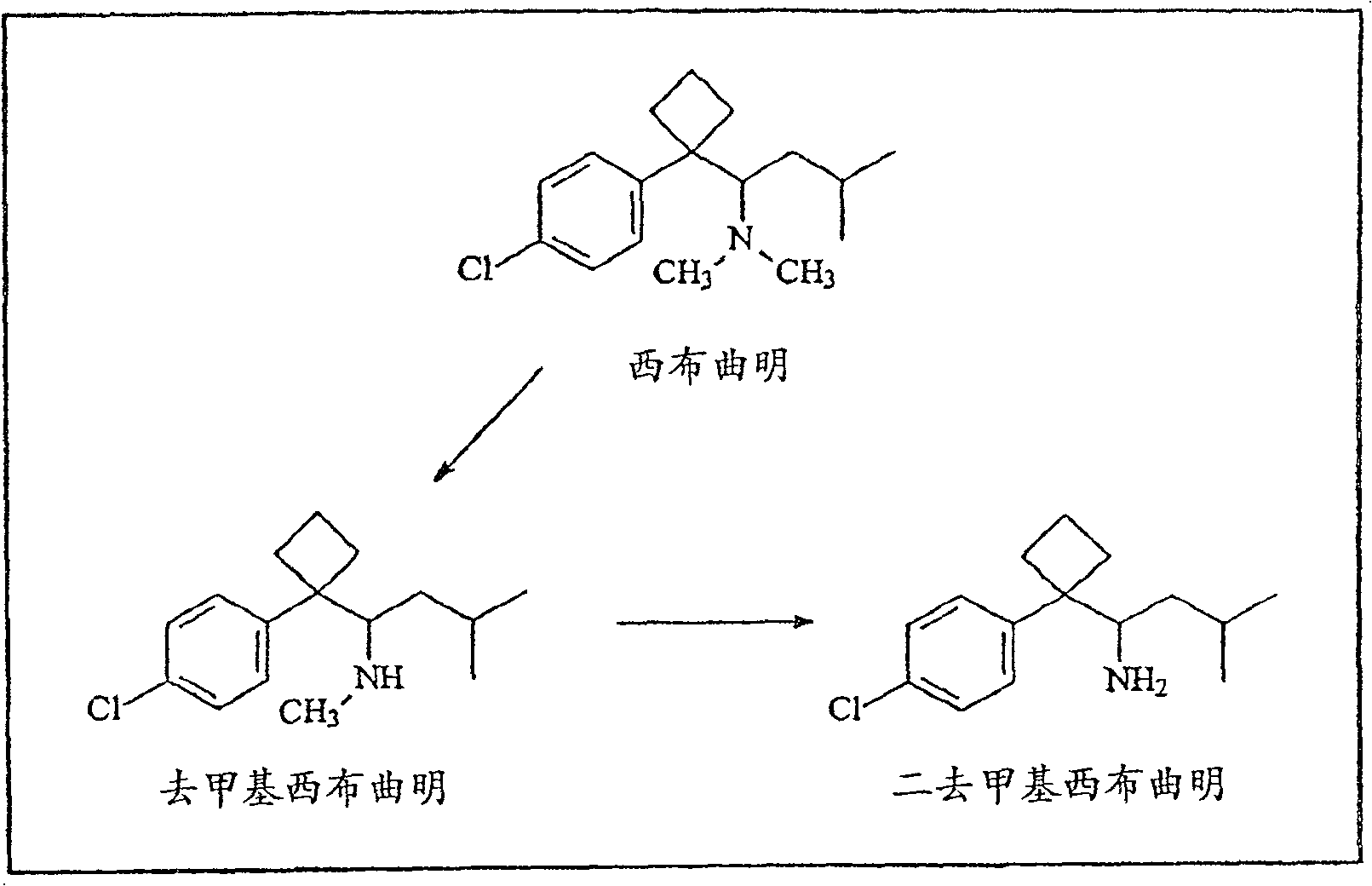
[0160] 5.1. Example 1: Synthesis of Sibutramine
[0161] Synthesis of 1-(4-Chlorophenyl)cyclobutanenitrile
[0162] To a suspension of NaH (176 g 60%, washed with hexane) in dimethyl sulfoxide (150 mL) was added chlorobenzylnitrile (303 g) over a period of 1 hour at room temperature with mechanical stirring and 1,3-dibromopropane (223 mL, 445 g). The reaction mixture was stirred for another 1 hour and isopropanol (10 ml) was slowly added to quench excess NaH. Water (150 mL) was added. The reaction mixture was extracted with tert-butyl methyl ether (MTBE) (2x200 mL), and the combined extracts were washed with water (3x200 mL), brine, and washed over MgSO 4 dry. The solvent was removed with a rotary evaporator and the final product was purified by distillation to give the title compound (22 g, 56%) as a light yellow oil, bp 110-120°C / 10 mmHg. pass the product through 1 H NMR assay.
[0163] Synthesis of 1-[1-(4-chlorophenyl)cyclobutyl]-3-methylbutylamine
[0164] A solut...
Embodiment 4
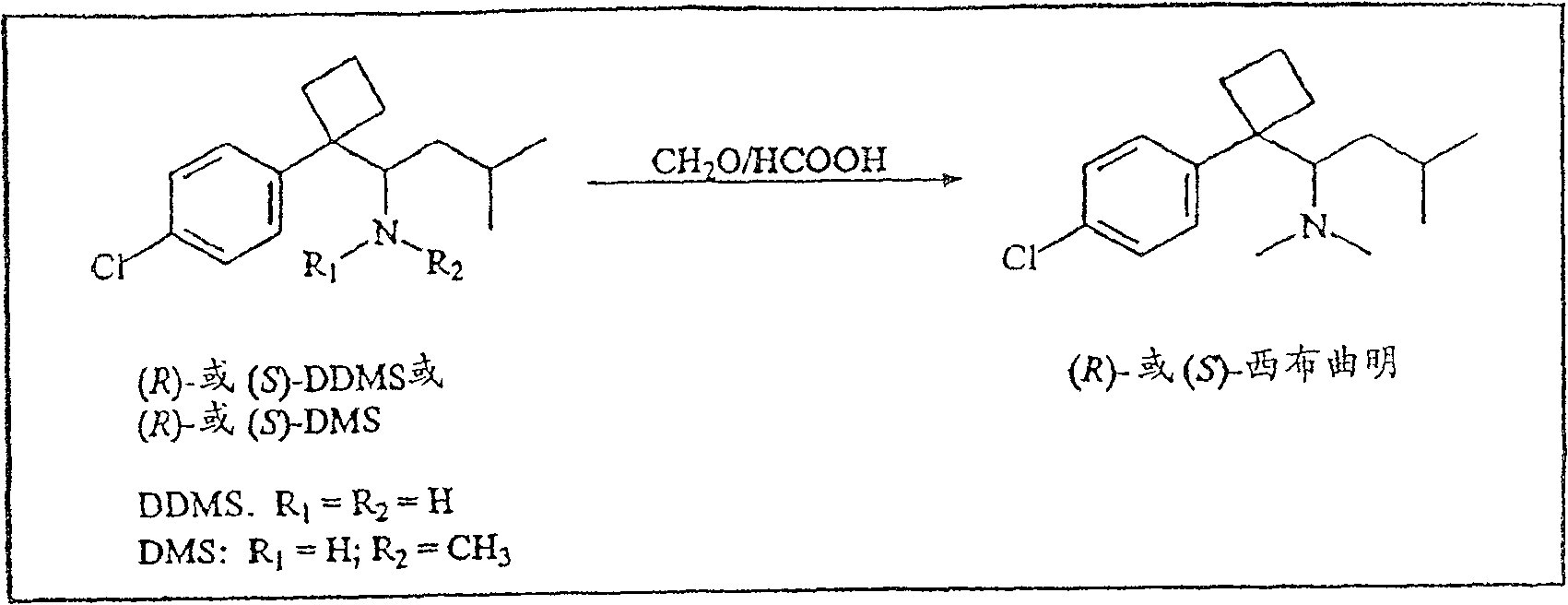
[0176] To a solution of (-)-desmethylsibutramine (0.78 g) in ethyl acetate (5 mL) was added HCl / diethyl ether (1M, 5 mL) at 0°C. The reaction mixture was stirred for 1 hour and the solid was collected by filtration. The solid was then dried to yield 068 g of a white solid. through 1 H and 13 CNMR (DMSO-d 6 ) assayed the product and determined >99% chemical purity by HPLC. [α]=-5°(c=05, H 2 O). The racemate and other enantiomers were prepared and determined in the same manner. 5.4. Example 4. (R / S)-desmethylsibutramine
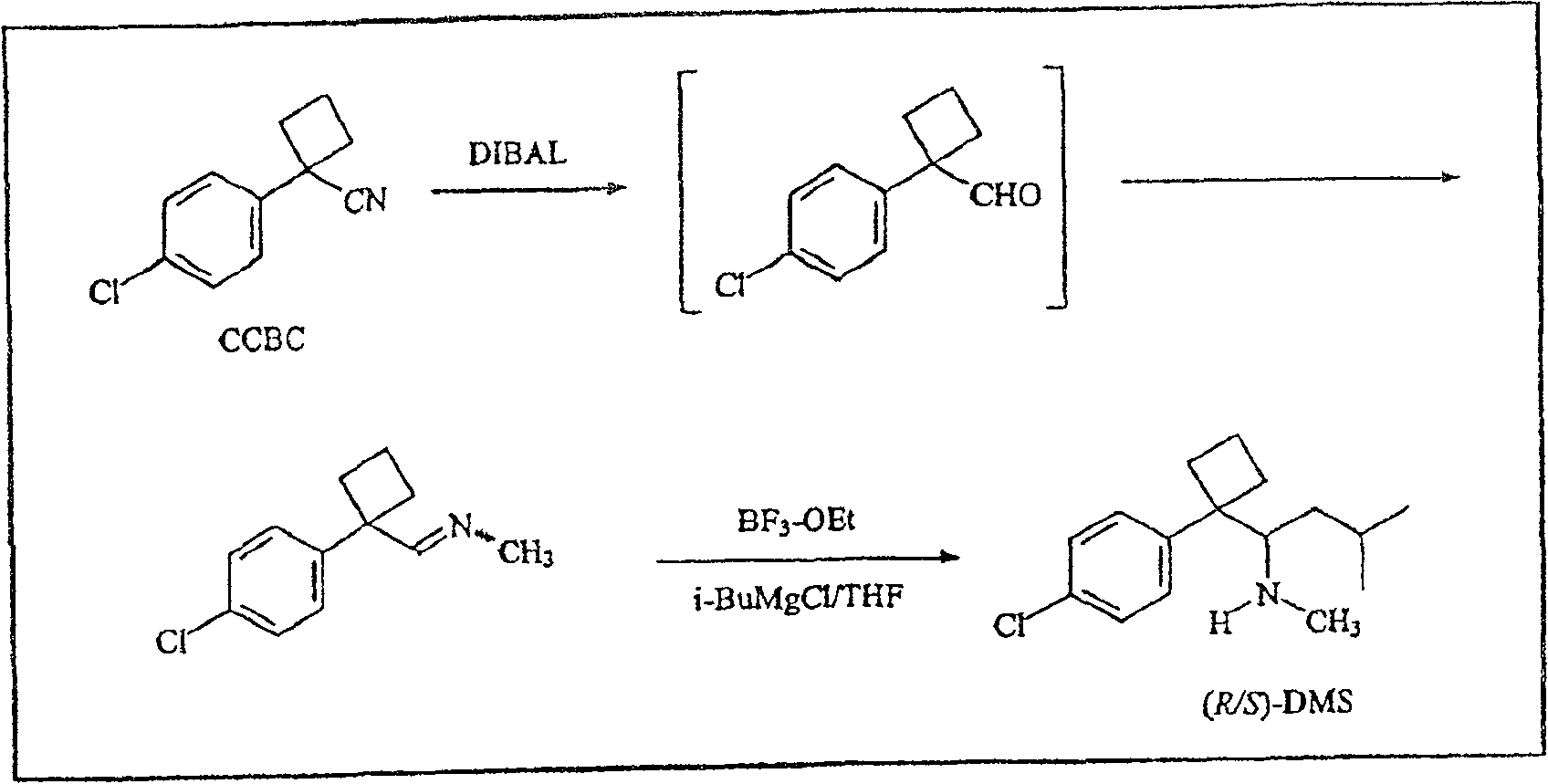
[0177] Another method for the preparation of racemic desmethylsibutramine ((R / S)-DMS) is shown in Scheme 2 and described in detail below
[0178]
[0179] Process 2
[0180] Preparation of 1-(4-chlorophenyl)-1-cyclobutylcarbaldehyde
[0181] Following Scheme 2, diisobutylaluminum hydride (DIBAL-H) (87 mL, 1 M in THF, 87.0 mmol) was added to 1-(4-chlorophenyl)cyclobutyronitrile ( CCBC; 10g, 521mmol) in solution. The resulting mixture was stirred...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com