Treatment of tauopathies
A protein and phosphorylation technology, applied in the field of treatment of tau protein disease, can solve the problem of limited selection of tau protein disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0360] Materials and methods
[0361] mouse
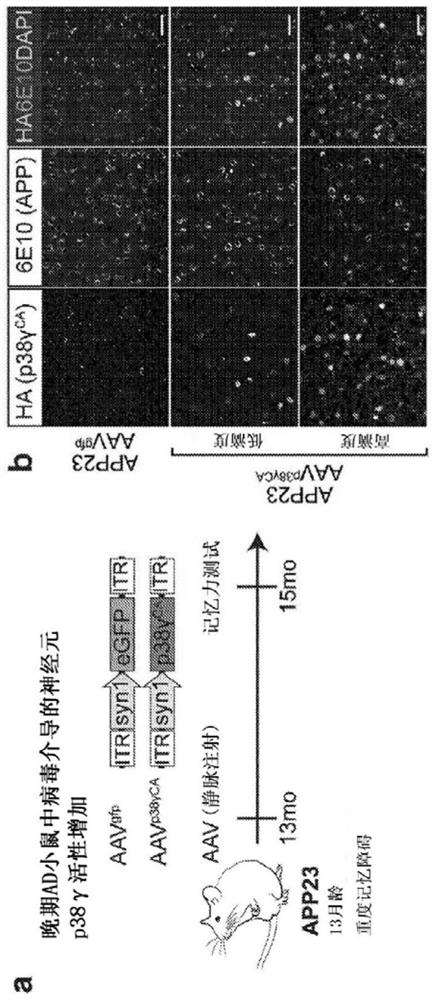
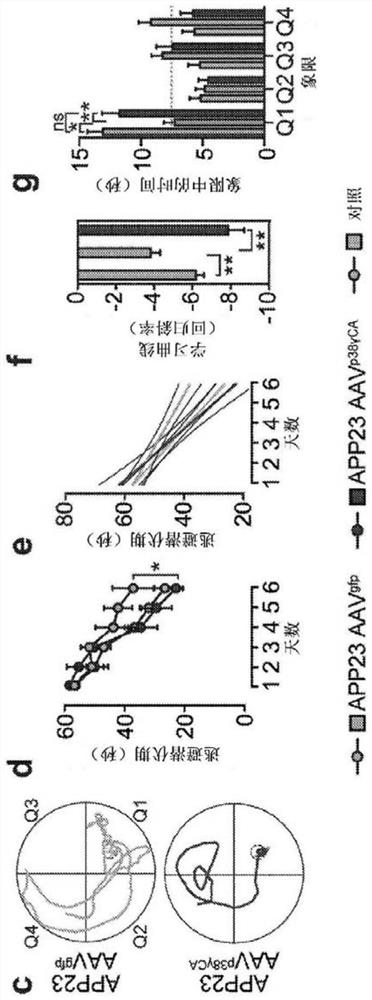
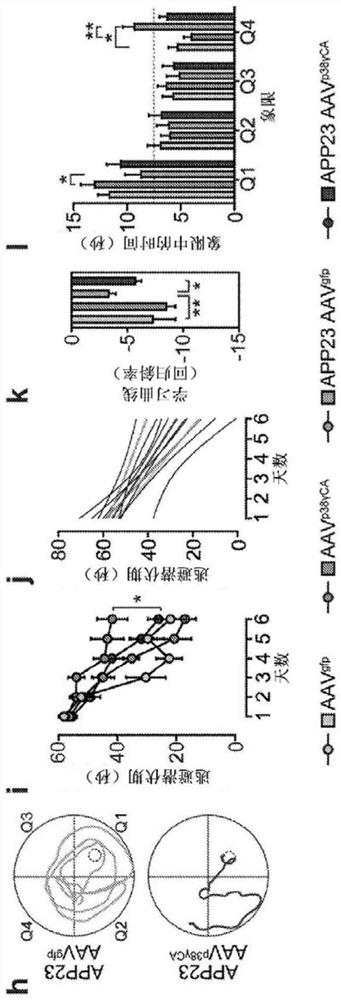
[0362] APP23 mice expressing human K670N / M671L mutant APP in neurons (Sturchler-Pierrat et al., 1997), ALZ17 mice expressing human non-mutated tau in neurons (Probst et al., 2000), tau - / - (Tucker et al., 2001), p38γ - / - {Perdiguero, 2007#164} mice, transgenic mice expressing constitutively active p38γCA in neurons (Ittner et al., 2016) and transgenic Tau58 mice expressing P301S mutant human tau in the brain (van Eersel et al. ., 2015) have been described previously. All mouse lines were maintained on the C57BI / 6 background. Animal experiments were approved by the UNSW Animal Ethics Committee. Mice were genotyped by polymerase chain reaction using isopropanol-precipitated DNA from tail biopsies as template. Oligonucleotide primers for genotyping target alleles and transgenes by polymerase chain reaction (PCR) are described in Table 1.
[0363] genome editing
[0364] The mouse Mapt gene was targeted using CRISPR / Cas9 as prev...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com