Nav1.7 antibodies and methods of using the same
A nav1.7, antibody technology, applied in chemical instruments and methods, antibodies, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve problems such as poor display selectivity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2-12
[0549] Materials and methods for Examples 2-12
[0550] Antibody production and purification. Mouse monoclonal antibodies were generated by Abmart using peptides having the following sequences: HHPMTEEFKN (115 (also referred to herein as CTmab); SEQ ID NO: 20) and VELFLADVEG (1E16 (also referred to herein as SVmab1); SEQ ID NO: 21). Both antibodies are IgG1. Upon receipt of the hybridomas, multiple rounds of limiting dilution cloning are performed to select for stable monoclonal cell populations. Using recombinant Na V 1.7 Enzyme-linked immunoassay (ELISA) assay of DIIVSD was used for screening. The hybridoma cells of 1E16 and 1I5 were incubated in a hollow fiber bioreactor (Fibercell Systems Inc, US), and then the supernatant was collected every two days. Supernatants from each harvest were screened by ELISA assay and purified on protein G sepharose columns following the manufacturer's protocol (Invitrogen, US).
[0551] Whole-cell patch-clamp recordings of HEK293 cells....
Embodiment 2
[0574] Monoclonal Antibody (mAb) Production
[0575] According to bacterial Na V Channel Na V For the crystal structure of Ab, the choice is equivalent to Na V1.7 The peptide of the tip (ring) of the DII voltage sensor switch (i.e., the S3-S4 loop) instead of using the complete channel as the antigen for antibody production ( Figure 4 B). Also choose the equivalent of Na V The peptide of the DIIS1-S2 loop of 1.7 was used as a negative control, because the S1-S2 loop does not move when the membrane potential changes, so the mAb binding to the S1-S2 loop will not significantly affect the Na V 1.7 channel gating ( Figure 4 C). One mAb was raised against each of the following regions: 1E16 mAb (i.e. sodium channel voltage sensor mAb (hence also referred to herein as SVmab1)) recognizes Na V The S3-S4 loop of 1.7, and the control 1I5 mAb (also referred to herein as CTmab) recognize Na V 1.7 S1-S2 ring. Both antibodies belonged to the same subtype and showed positive ELIS...
Embodiment 3
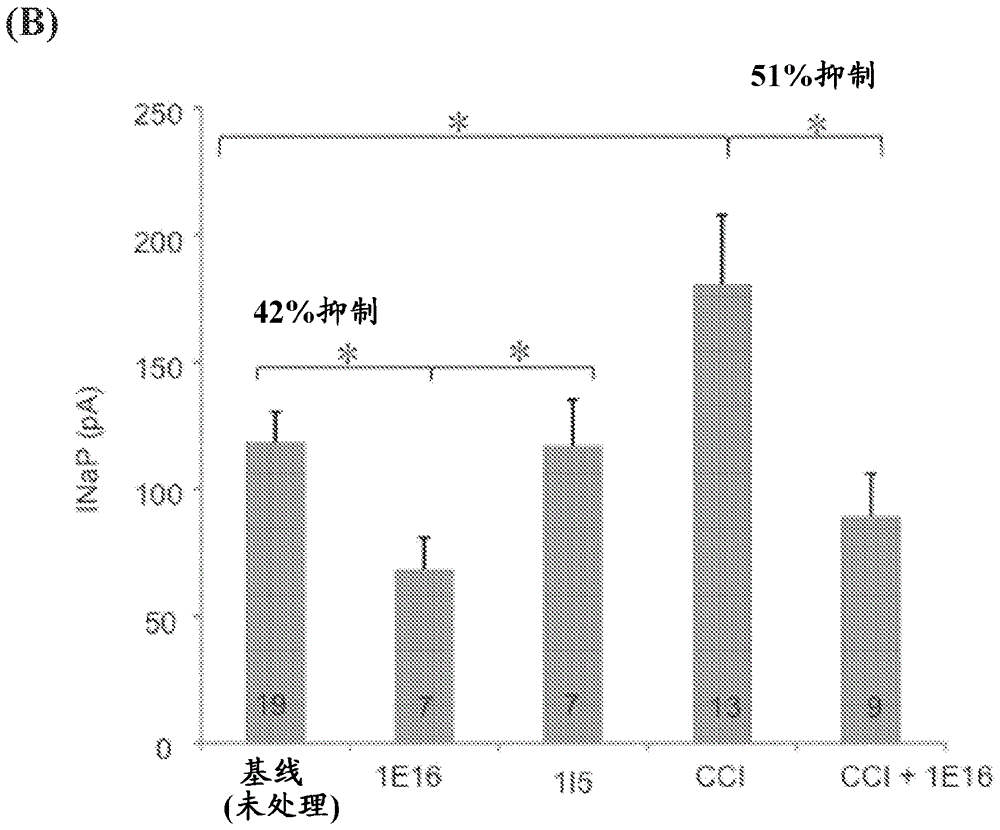
[0577] 1E16mAb stabilizes Na in an application (state)-dependent manner V 1.7's closed state
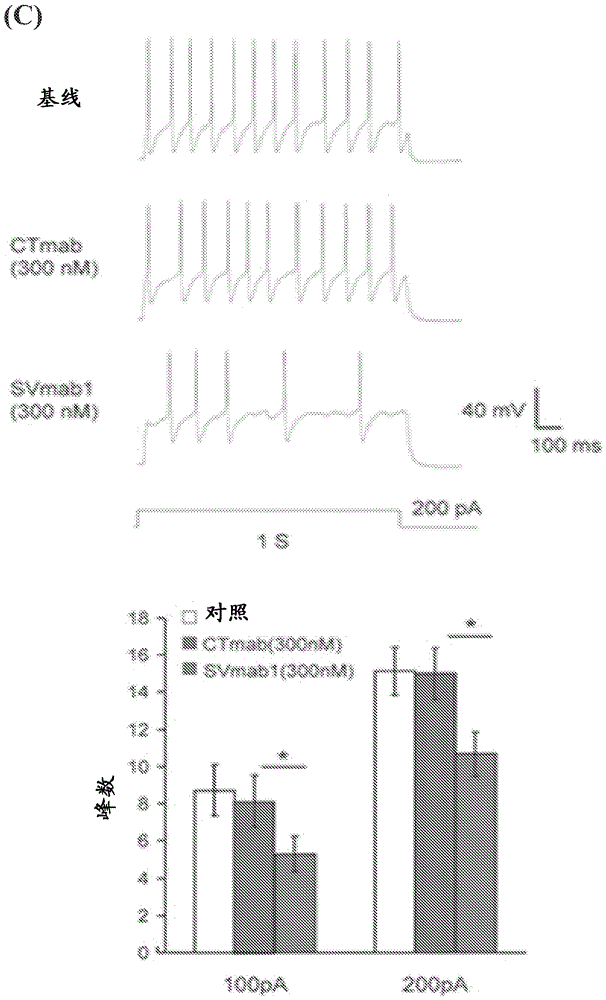
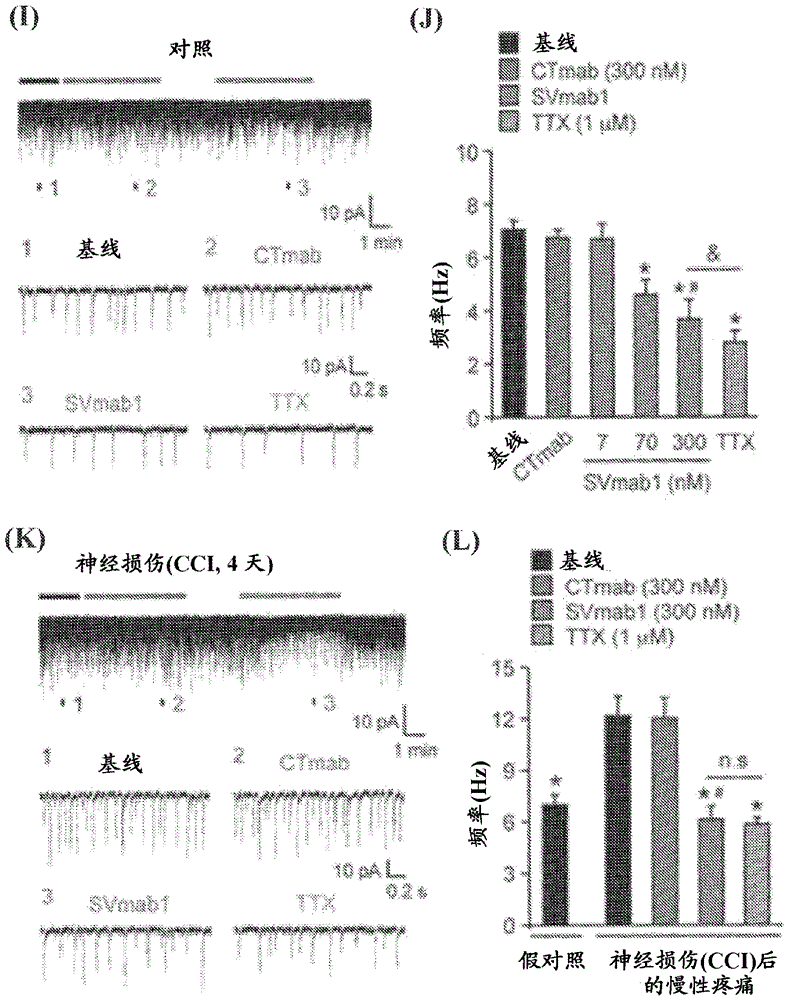
[0578] To test the 1E16 and 1I5mAbs against Na V 1.7 Effect of Transiently Expressed Na Using a Whole-Cell Voltage Clamp Configuration V 1.7 HEK293 cells were subjected to electrophysiological recording (ie, patch clamp recording). Current traces were elicited by 30 ms voltage steps between −80 and +60 mV in 10 mV increments from a membrane clamping potential of −120 mV. When 1 μM 1I5 mAb was added to the extracellular side, no significant change was observed in the peak sodium current ( Image 6 A and 6B). However, 100 nM 1E16 mAb produced a significant reduction in peak sodium current ( Image 6 C and 6D). Comparison of the conductance-voltage relationship of the control 1I5mAb- and 1E16mAb-improved currents when 100 nM 1E16mAb was added showed a shift in depolarization (approximately 20 mV) ( Image 6 E). In contrast, comparison of the steady-state inactivation curves show...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com