Analgesic and antipruritic pharmaceutical composition and application method thereof
A composition and drug technology, applied in the field of medicinal chemistry, can solve problems such as unclear, poor effect, and poor analgesic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example of the effect of giving a single drug
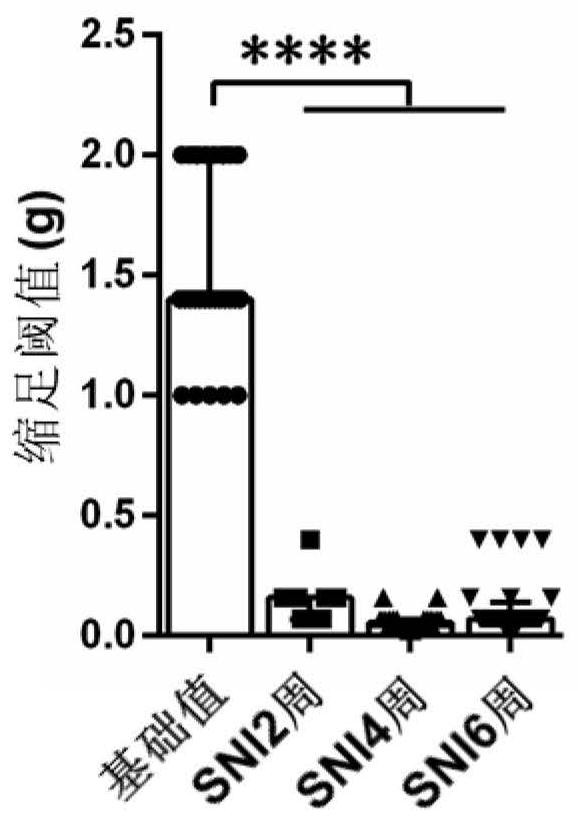
[0035] To test Na v 1.7 and Na v 1.8 The effect of inhibitors on relieving neuropathic pain induced by peripheral nerve injury at different time points. We purchased 5-6 week-old C57 / BJ6 mice (Shanghai Slack Experimental Animal Co., Ltd.), and fed them for 6 months. The mouse was placed in a transparent plexiglass cover on an iron frame, and the basic mechanical threshold (paw withdrawal threshold, PWT) of the mouse was detected with Von Frey (DanMicGlobal, CA, USA) after 30 minutes. The average threshold is 1.54g ± 0.07g, see figure 1 . The neuropathic pain C57 / BJ6 mouse model of Spared nerve injury (SNI) nerve injury was prepared according to the references, and the mechanical pain threshold of the mice was also detected by VonFrey at 2 weeks, 4 weeks, and 6 weeks after the operation, and the threshold value was significantly Below the preoperative threshold, the average thresholds were 0.14g±0.02g, 0.06g±0.01g, 0.12...
Embodiment 3
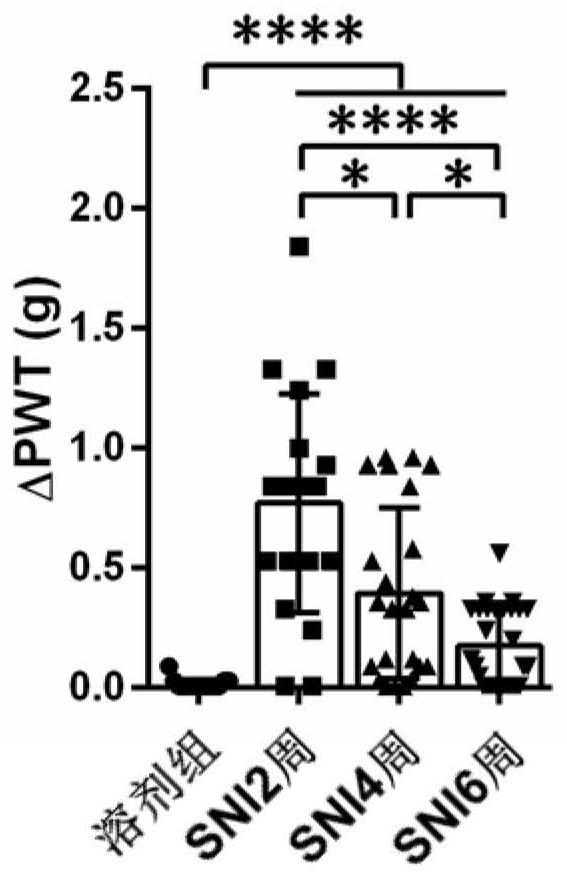
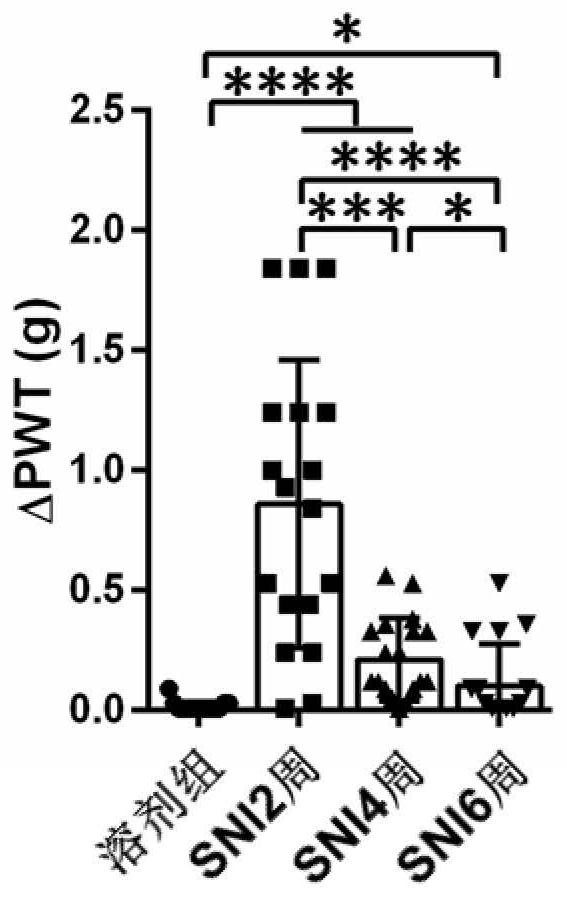
[0045] In order to find the best analgesic Na v 1.7 and Na v 1.8 combinations of inhibitor classes, we tested another Na v 1.7 The analgesic effect of the combination of inhibitors GNE-0439 and PF-04885614. After intraperitoneal injection of 20 μg / kg GNE-0439 and 90 μg / kg PF-04885614 into mice 6 weeks after SNI, Von Frey was used to detect the mechanical threshold of the mice one hour later, and it was found that the combination of GNE-0439 and PF-04885614 obtained the sedation The analgesic effect was not significantly better than the analgesic effect of GNE-0439 alone (△PWT=0.77g±0.14g, see Figure 9 ), and the analgesic effect (△PWT=1.38g±0.15g) was not as good as the combined administration of 2mg / kg PF-05089771 and 90μg / kg PF-04885614. Then we tested another Na v1.8 Analgesic effect of combined use of inhibitors PF-04531083 and PF-05089771. After intraperitoneal injection of 2mg / kg PF-05089771 and 10mg / kg PF-04531083 to mice 6 weeks after SNI, the mechanical threshol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com