Phospho-specific antibody that recognizes tau
An antibody and specific technology, applied in the direction of antibodies, antibody medical components, specific peptides, etc., can solve unmet problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0507] Example 1: Generation and screening of hybridomas and antibodies
[0508] The purpose of this study was to generate and screen anti-Tau mAbs (monoclonal antibodies). Hybridomas were generated by fusion of the spleen of a Tau vaccine immunized mouse with a myeloma cell line. The reactivity of hybridomas to phosphorylated and unphosphorylated full-length Tau proteins and phosphorylated and unphosphorylated Tau antigenic peptides used in vaccine preparation was evaluated. Hybridoma screening was also performed on Tau transgenic mouse brain sections by the reactivity of hybridoma supernatants to Tau tangles using immunochemistry.
[0509] 1.1 Method
[0510] 1.1.1 Fusion
[0511] Wild-type C57BL / 6 mice inoculated with ACI-35 (Tau 393-408 [pS396, pS404]) were used for hybridoma production. Mice were boosted again with ACI-35 vaccine on day 0 and on day 4, and fusions were performed on day 7.
[0512] 6 × 10 from immunized mice 7 (ACI-35) splenocytes with 2 × 10 7 ...
Embodiment 2
[0531] Example 2: Cloning of antibody light and heavy chain variable regions
[0532] Antibody heavy and light variable region genes from hybridoma cells were cloned and DNA sequences and positions of complementarity determining regions (CDRs) and antibody binding characteristics were determined.
[0533] Using Qiagen RNeasy Mini Kit (Cat No: 74104) from 3 x 10 6Total RNA was prepared from each hybridoma cell (1 vial). RNA was eluted in 50uL of water and examined on a 1.2% agarose gel.
[0534] V preparation with IgG and kappa constant region primers using reverse transcriptase H and V K cDNA. The first strand cDNA was amplified by PCR using a larger set of signal sequence primers. The amplified DNA was gel purified and cloned into the vector T Easy (Promega). the obtained V H and V K Clones were screened for inserts of expected size. The DNA sequences of selected clones were determined bidirectionally by automated DNA sequencing. The positions of the complementa...
Embodiment 3
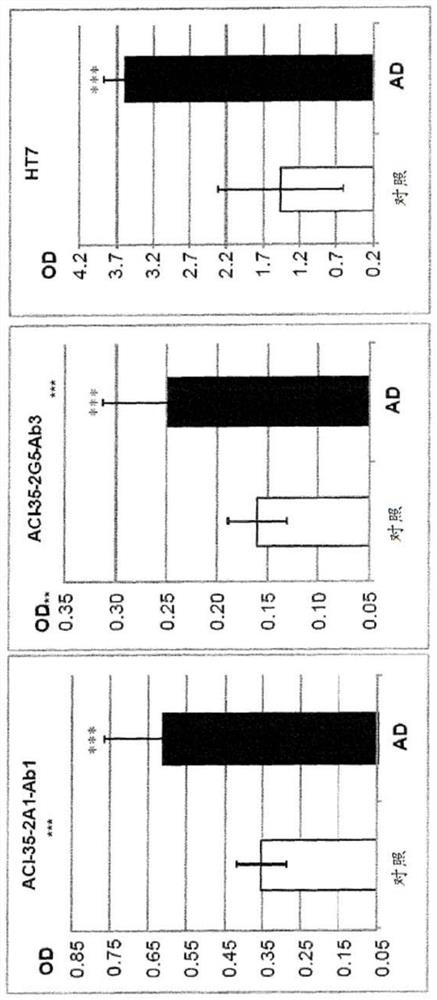
[0535] Example 3: Binding Study I
[0536] The objective was to measure phospho-Tau (pTau) binding of antibodies produced from subcloned hybridomas derived from mice immunized with Tau liposomal vaccine. To perform this test, an enzyme-linked immunosorbent assay (ELISA) was used to measure the binding of purified antibodies to phosphorylated and non-phosphorylated full-length Tau protein and to phosphorylated and non-phosphorylated Tau antigenic peptides for liposomal vaccine preparation combine.
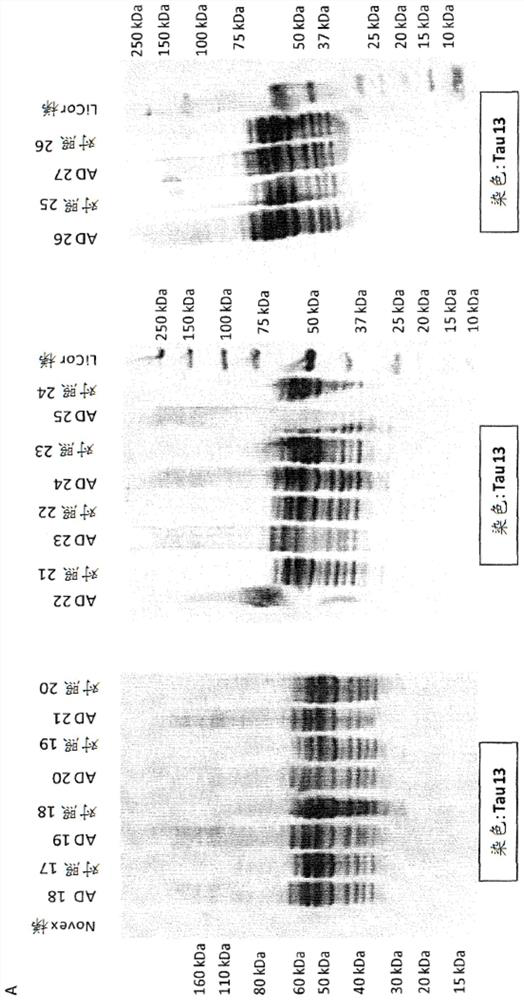
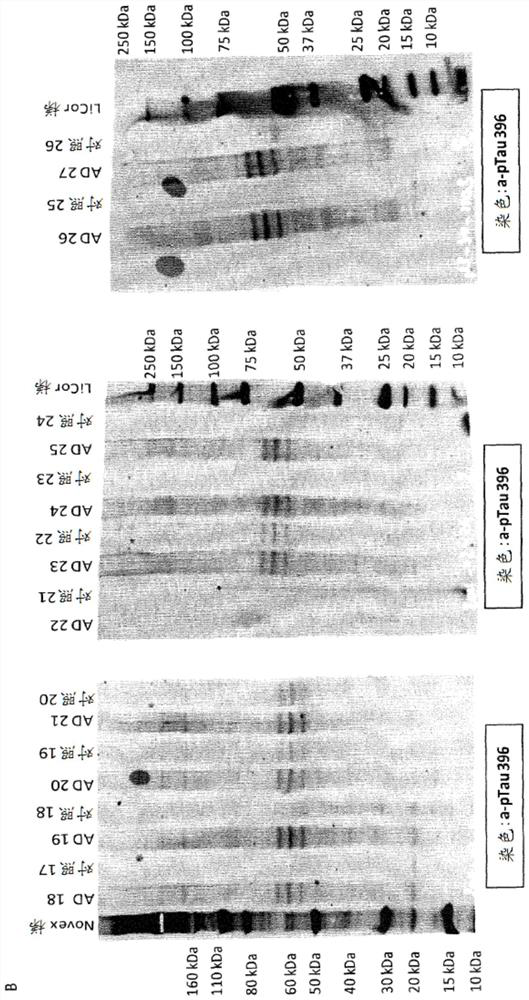
[0537] Screening is accomplished by two other methods. Immunohistochemistry (IHC) was performed on brain sections from Tau transgenic animals (TAUPIR) using an anti-Tau antibody as the primary antibody. Additionally, brain protein homogenates from Tau transgenic mice were immunoblotted (WB) using anti-Tau antibody as the blotting antibody.
[0538] 3. 1 method
[0539] 3.1.1 ELISA: Phospho-Tau binding assay
[0540] To test the binding of purified antibodies to Tau and pTa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com