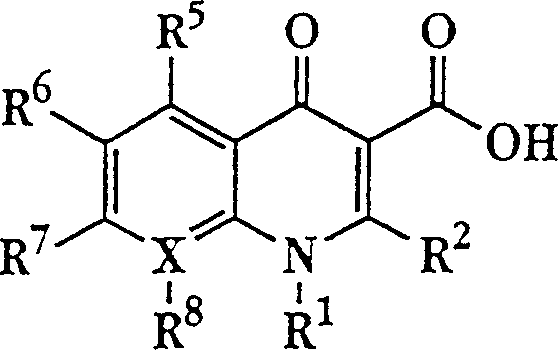
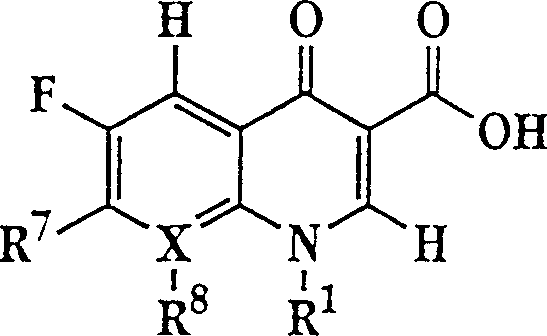
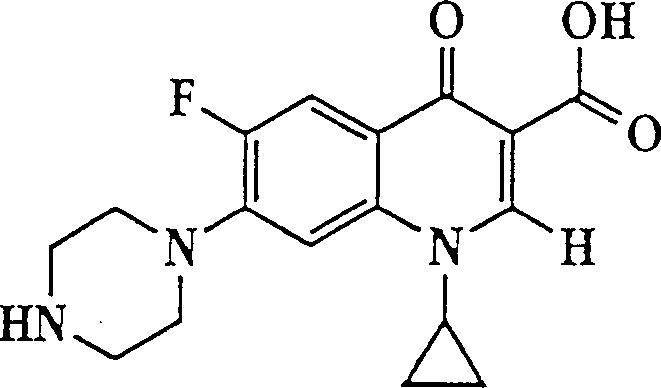
Liposome compositions for improved drugretention
A liposome composition and liposome technology, which are applied in the directions of liposome delivery, active ingredients of heterocyclic compounds, antibacterial drugs, etc., can solve the problems of high liposome leakage rate, release and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0095] Preparation of liposomes containing dextran sulfate-ciprofloxacin
[0096] A, the preparation of dextran sulfate ammonium salt
[0097] Dextran sulfate, sodium salt, molecular weight 8,000 Daltons (Dextran Product Limited (Ontario, Canada)) was converted to dextran sulfate ammonium salt as follows. An ion exchange column with a bed volume of 400 mL was packed with DOWEX 50X8-100 ion exchange resin (Aldrich Chemical Co, Milwaukee, WI). The packed column was flushed with 2L of 1N HCl at a flow rate of 3-4mL / min until the pH of the elution was about 1-2. Excess HCl was washed off the column with 2-3 L of deionized water until the pH of the elution water was approximately 5.5.
[0098] 300 mL of dextran sulfate sodium salt (50 mg / mL in water) was added to the resin and twelve 50 mL fractions were collected. Fractions containing dextran sulfate (fractions 3-12) were combined and the pH was adjusted from 1.29 to 5.5 with 5M ammonium hydroxide (approximately 25 mL).
...
Embodiment 3
[0108] Diafiltration of Dextran Sulfate against Saline Solutions
[0109] Dextran sulfate sodium salt (molecular weight 8,000 Daltons, Dextran Products Limited, Scarborough, Ontario, Canada) was dissolved in distilled water at a concentration of 100 mg / ml. With 300,000 molecular weight cut-off valve and 0.79ft 2 Surface area diafiltration cartridges (A / G / Technology Corporation, Needham, MA) were washed with 2 liters of distilled water. 90 mL of dextran sulfate was diafiltered against 1000 mL (approximately 10 volumes exchanged) of distilled water. The volume exchange liquid (each 100mL) was collected separately, and each 1mL was used for the quantification of dextran sulfate. The final residue was adjusted to the same initial volume (90 mL) with water, and then the content of dextran sulfate was determined.
[0110] The same diafiltration procedure was repeated using 0.15M NaCl, 0.35M NaCl, 0.45M NaCl and 1.0M NaCl. The permeate was collected and analyzed for dextran sul...
Embodiment 4
[0112] Liposome formulation with polyacrylic acid
[0113] An aqueous solution of 250 mM polyacrylic acid (Aldrich Chemicals, Milwaukee, WI) with a molecular weight of 2,000 Daltons and 50 mM ammonium sulfate in water was prepared and the pH of the solution was adjusted to 5.5 with 5M NaOH.
[0114] Liposomes were prepared as described in Example 2 by hydrating dissolved HSPC, cholesterol and mPEG-DSPE with an aqueous ammonium polyacrylate / ammonium sulfate solution in a molar ratio of 50:45.2:4.8. Unentrapped polymer was removed and ciprofloxacin was loaded into liposomes as in Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com