Patents
Literature
34 results about "Drugs retention" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
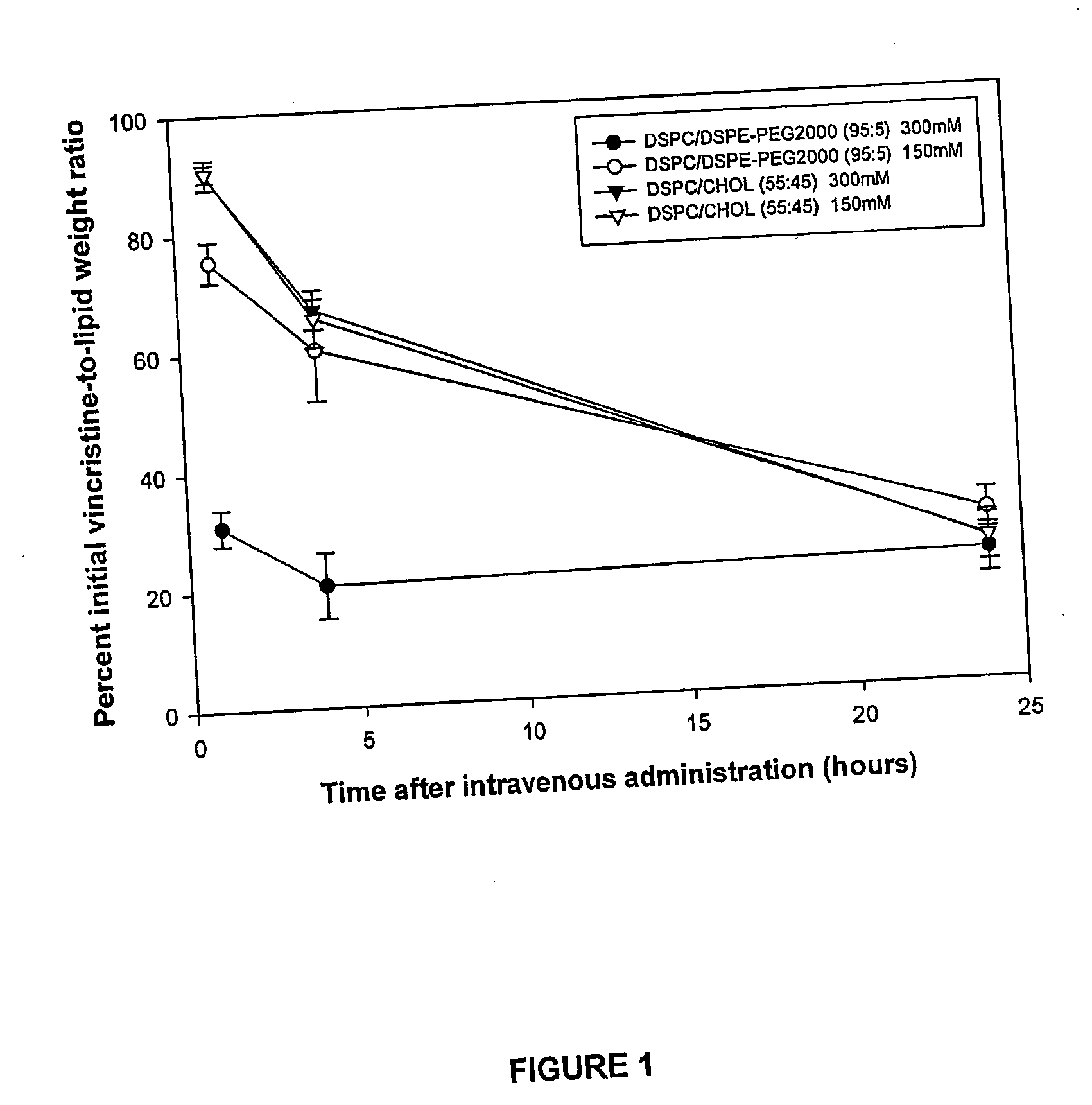
High drug:lipid formulations of liposomal-antineoplastic agents
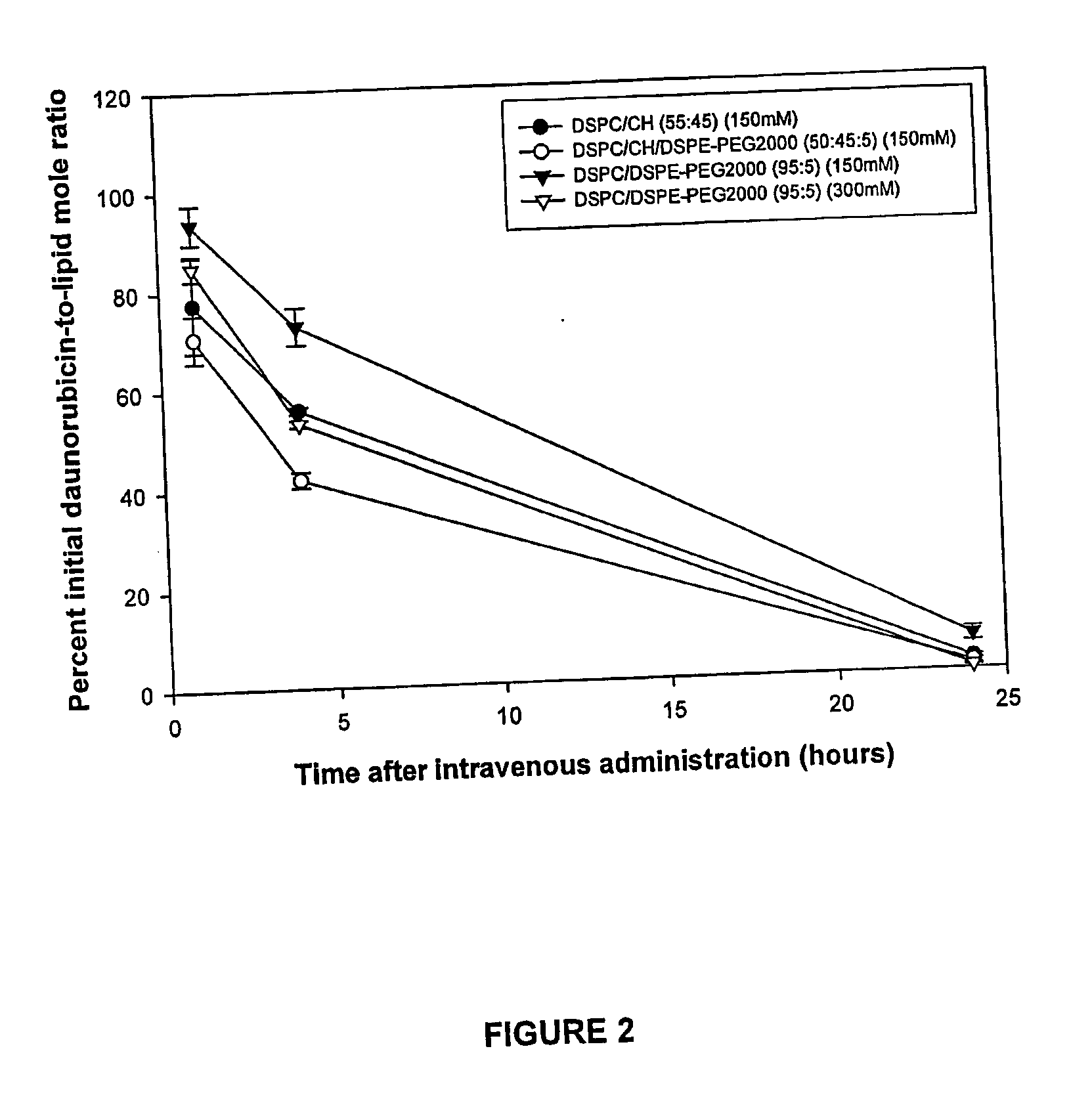
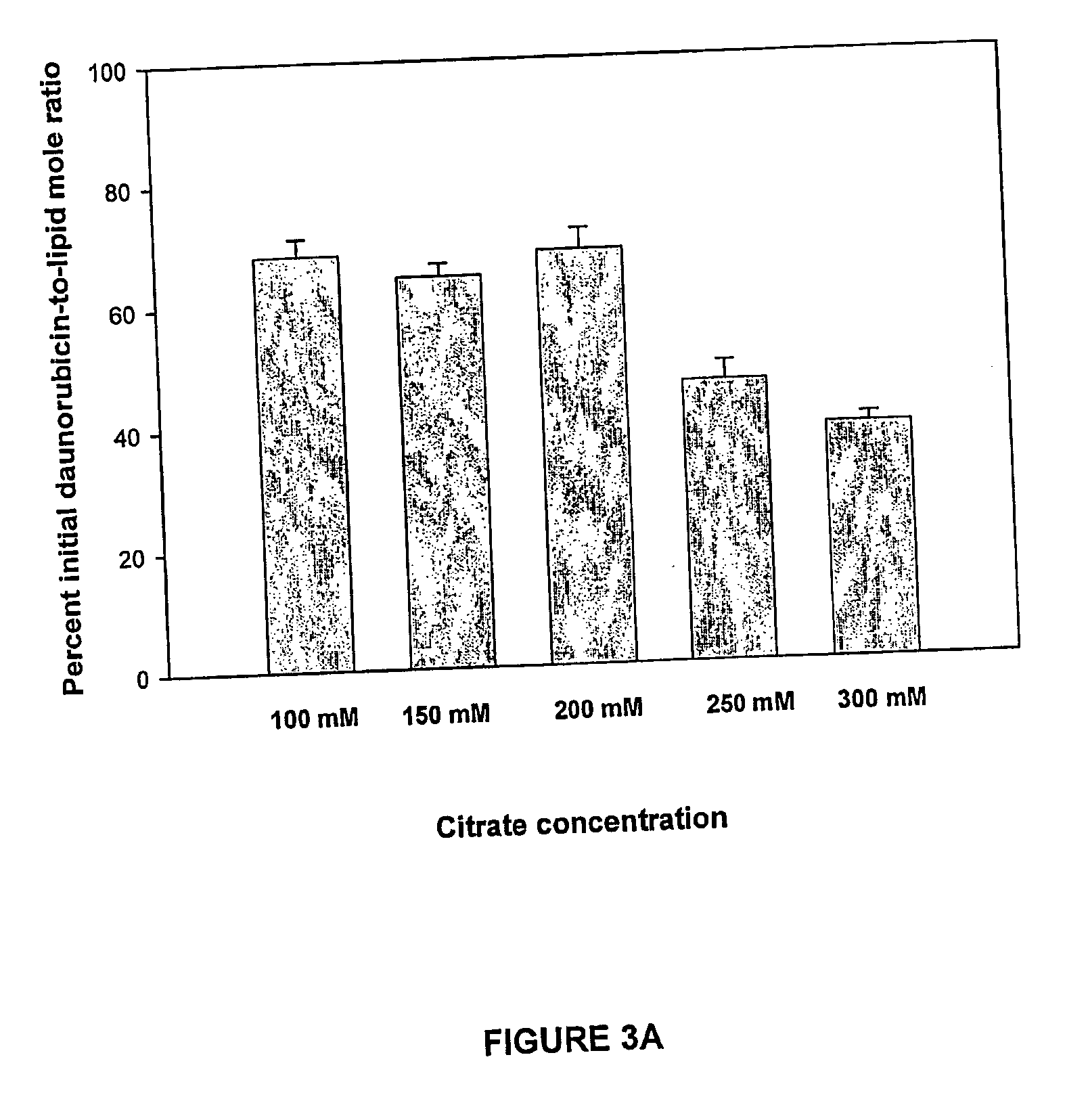
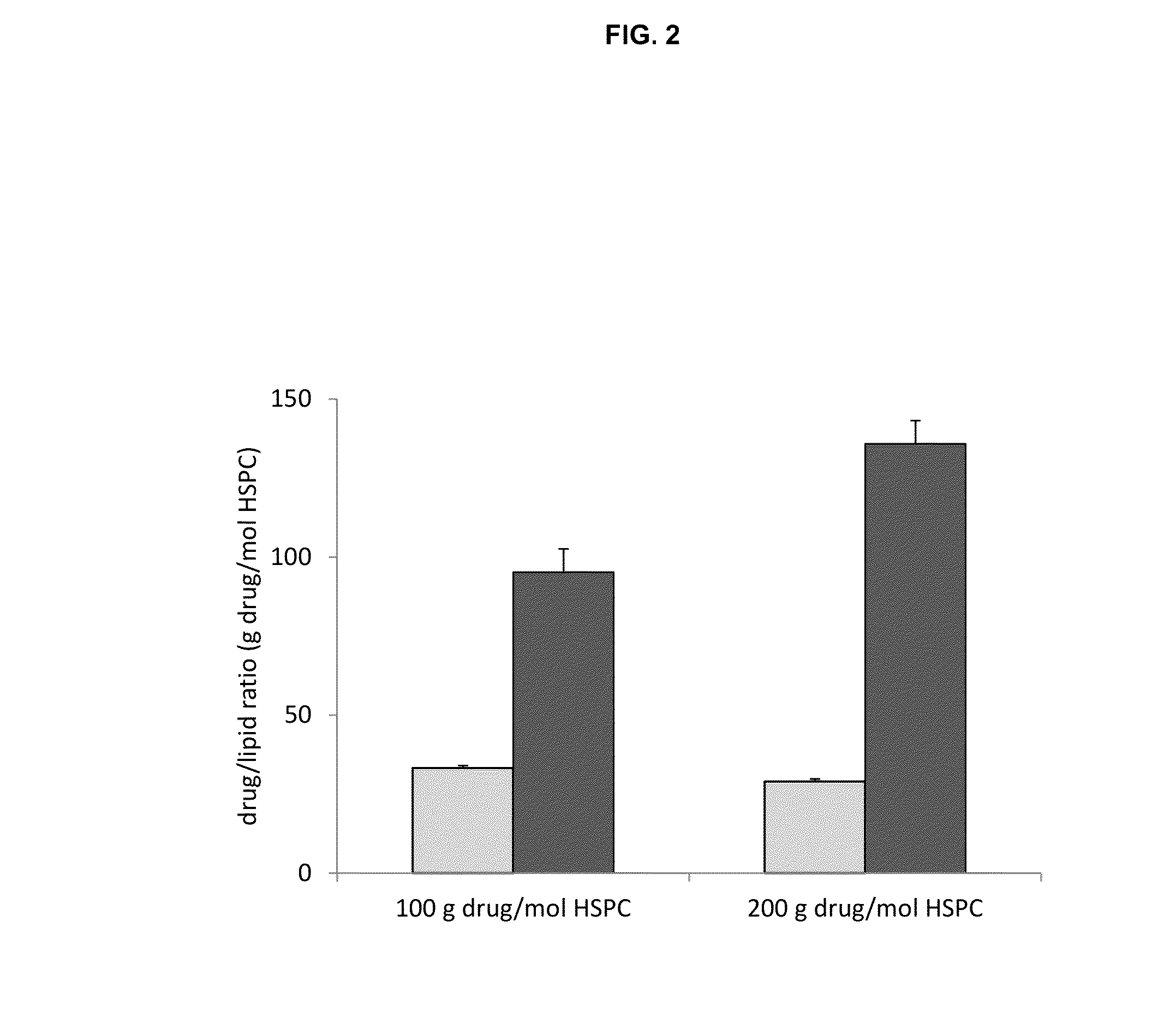
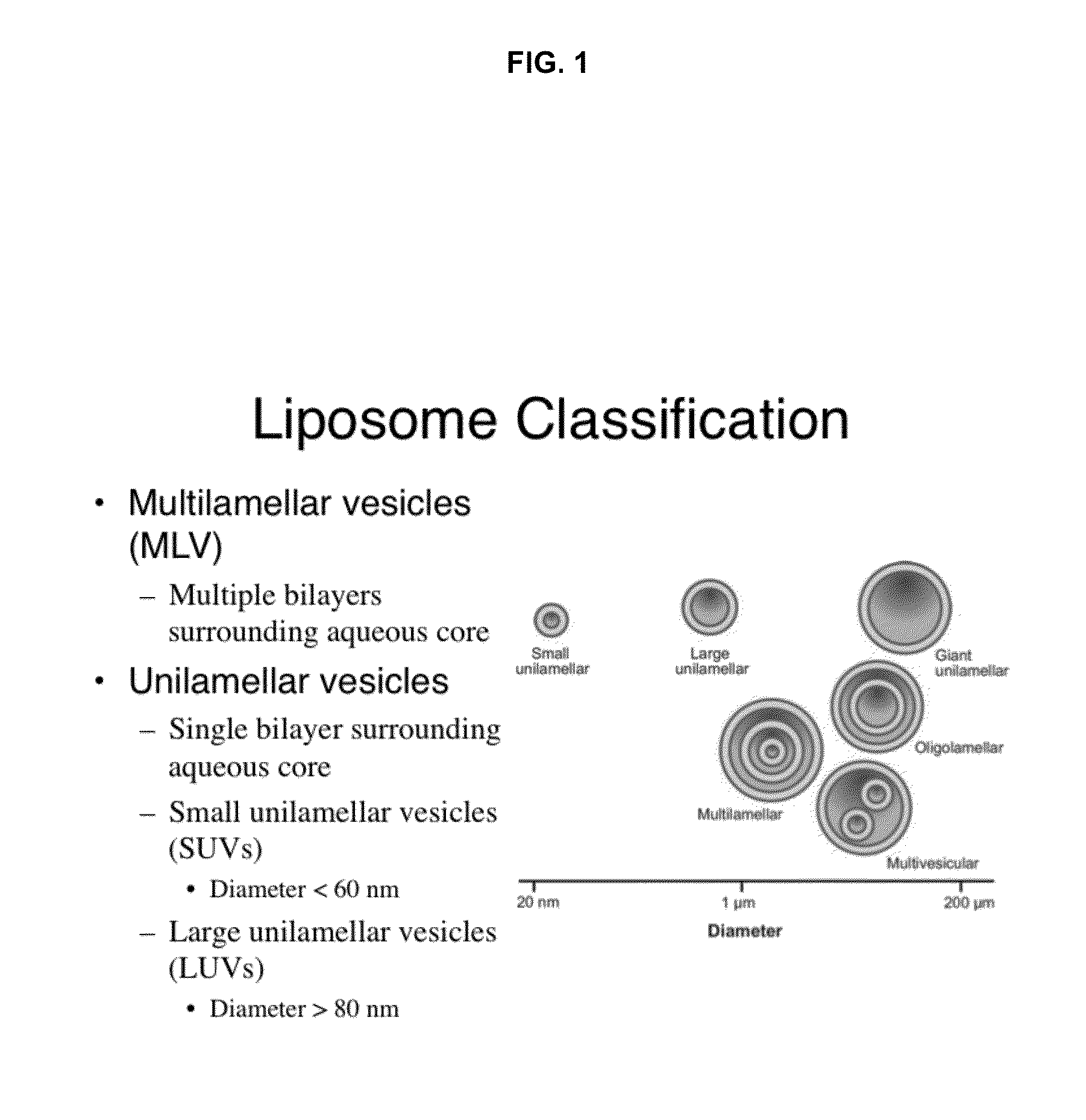
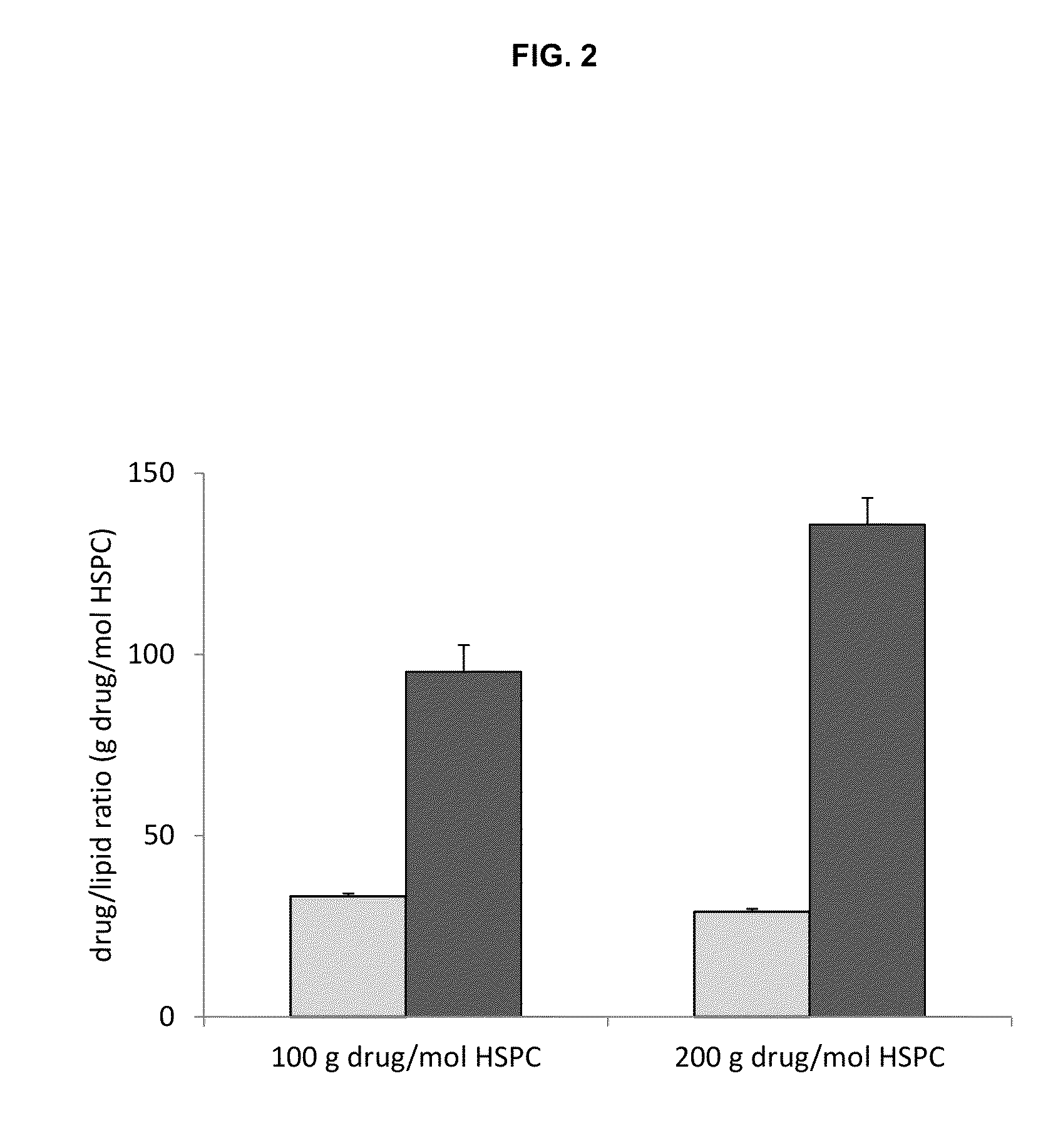
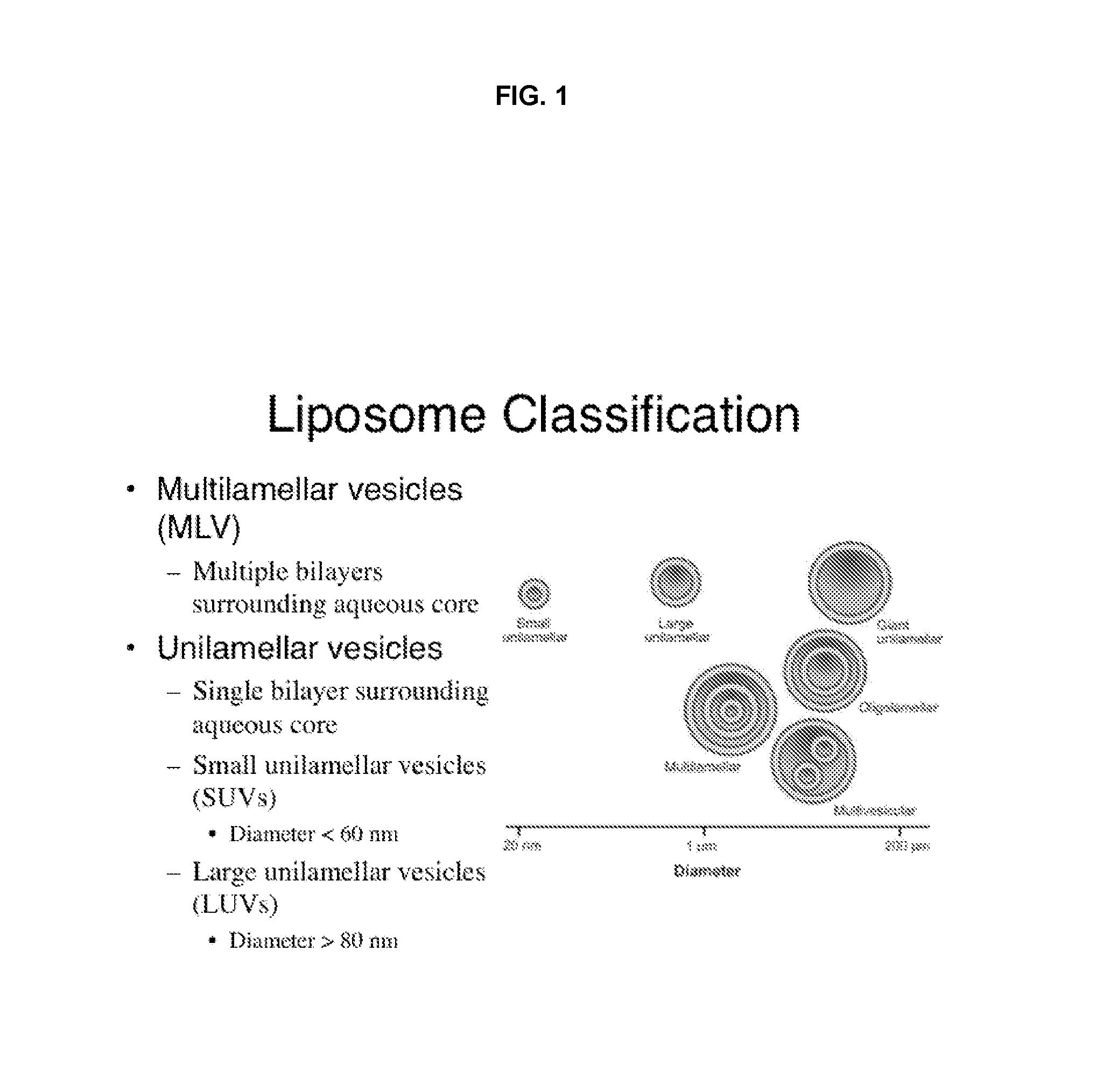
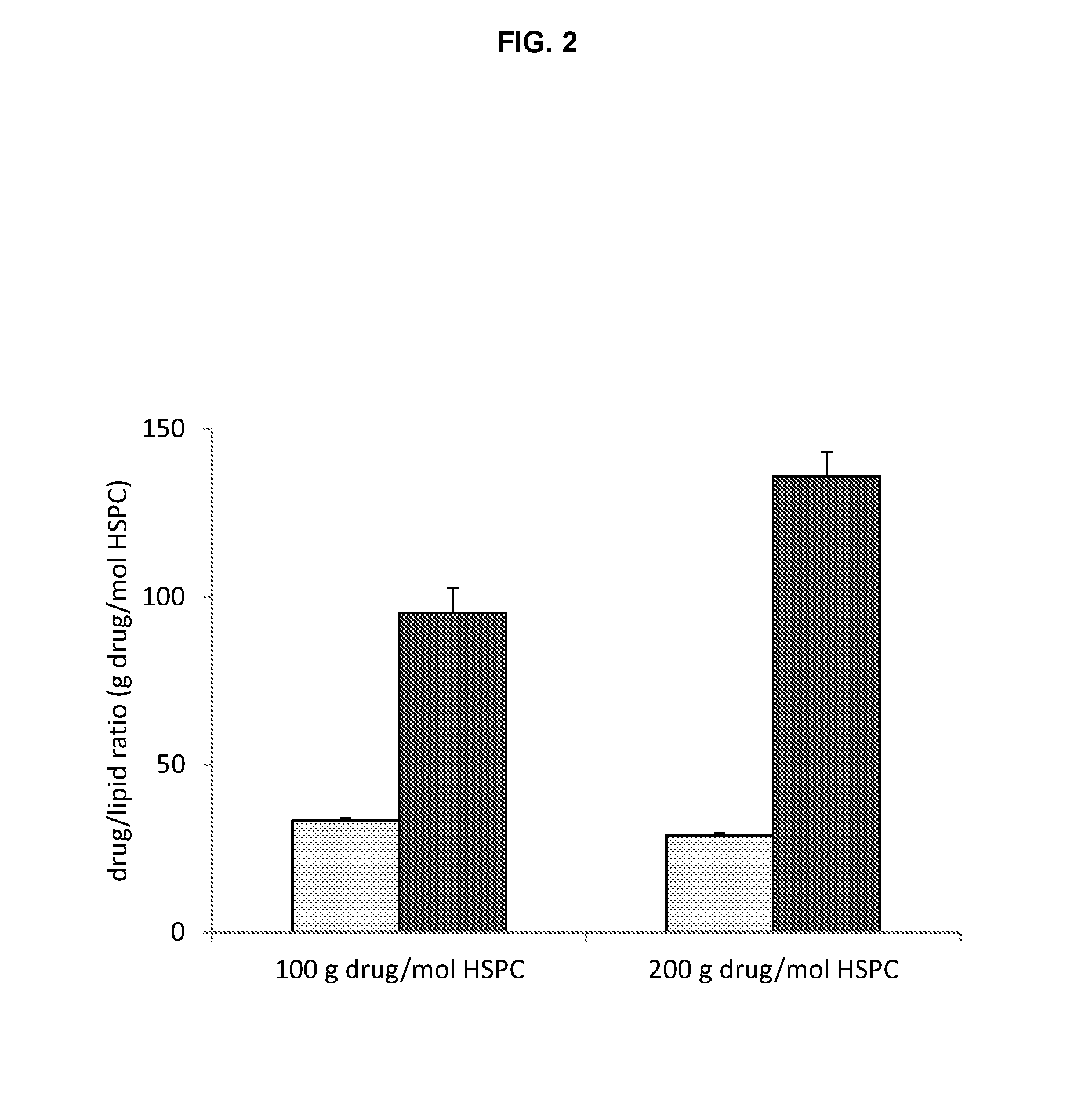
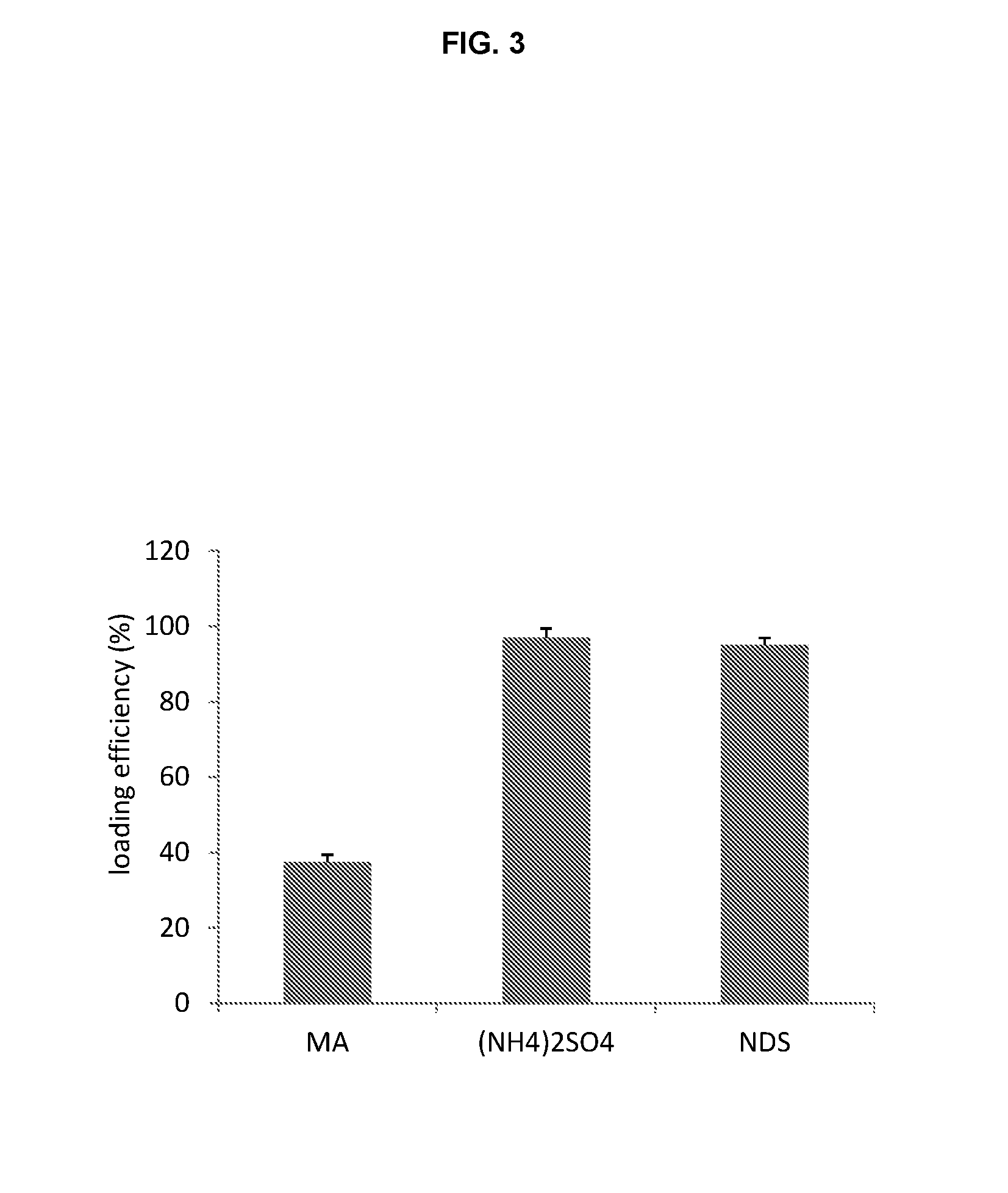
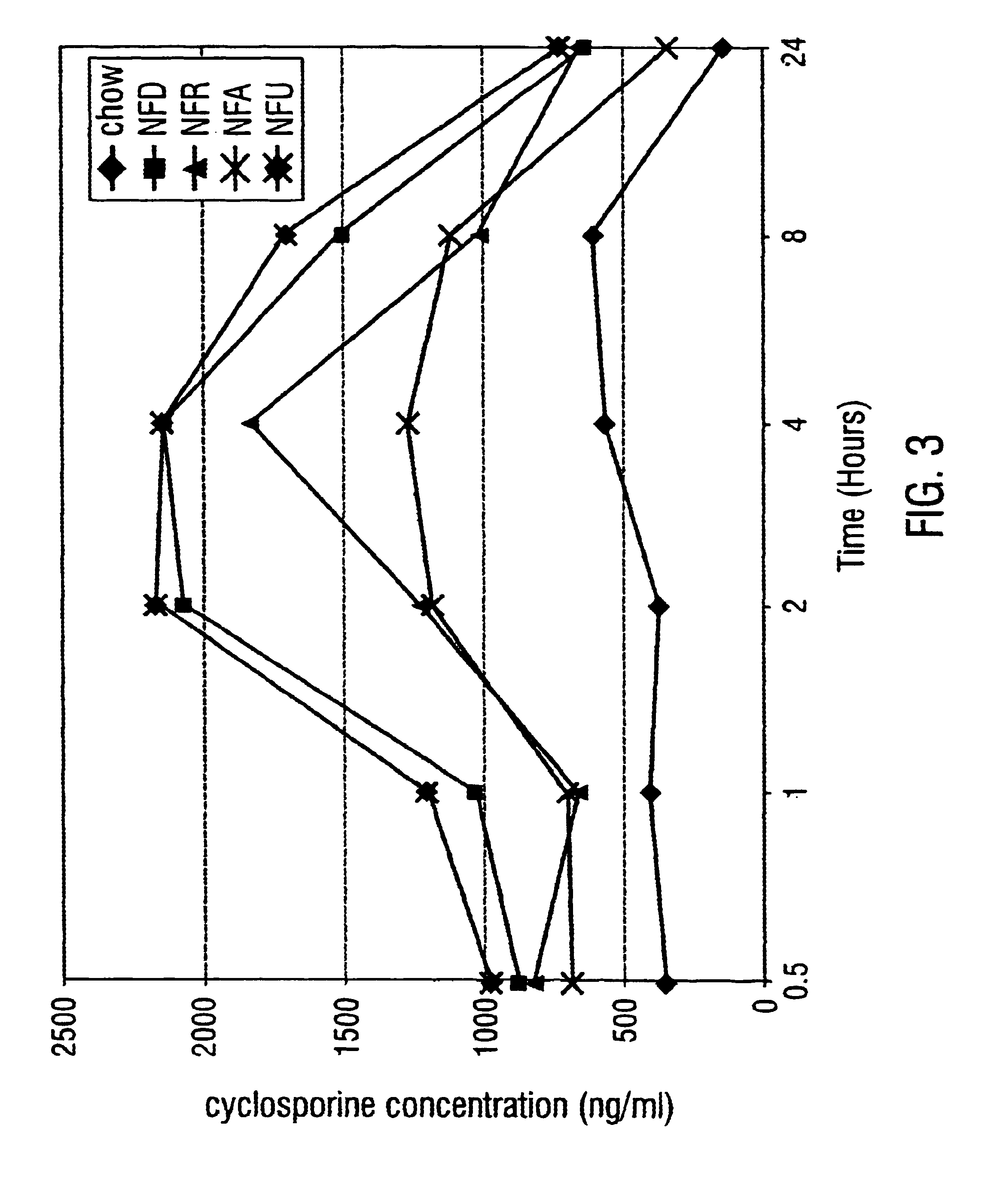
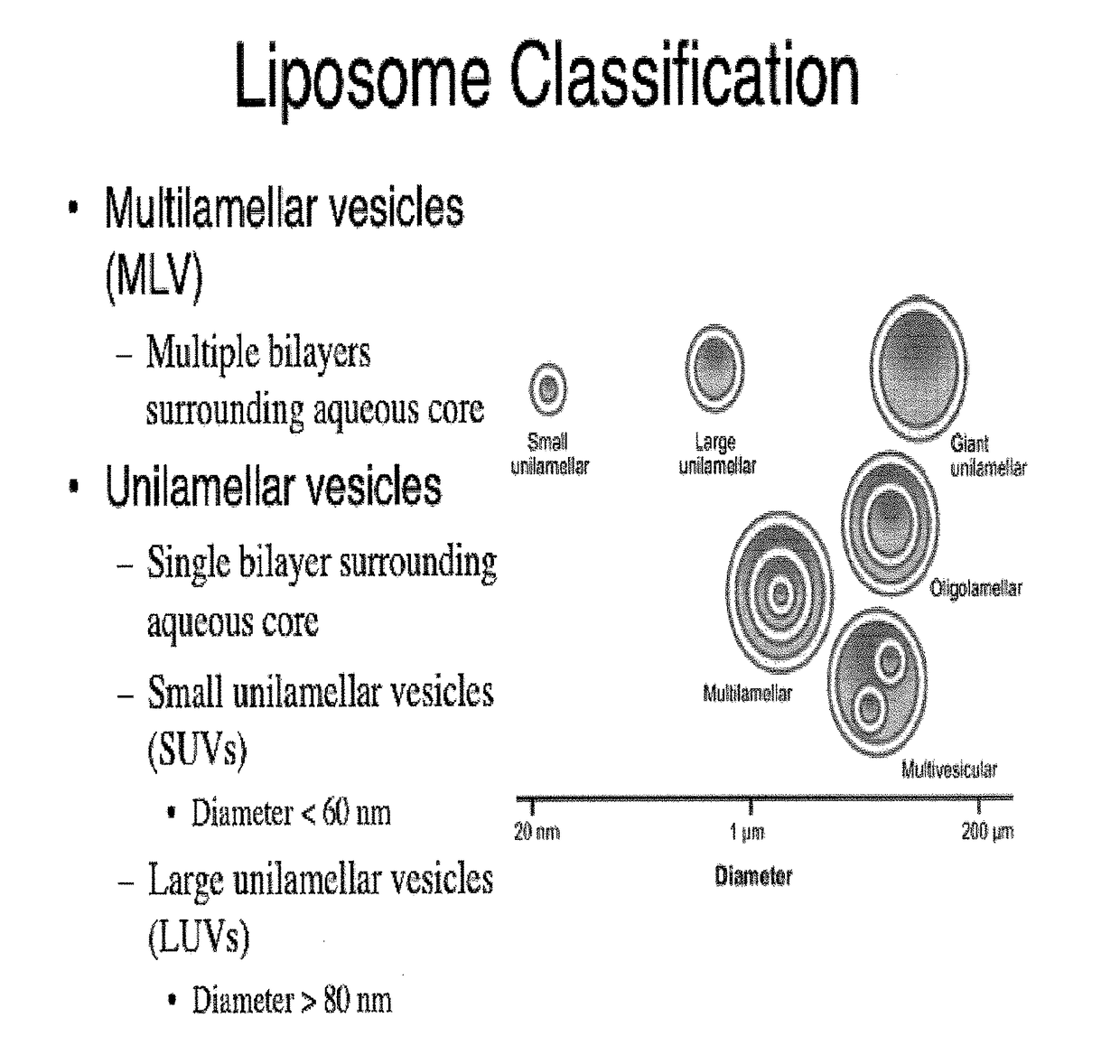
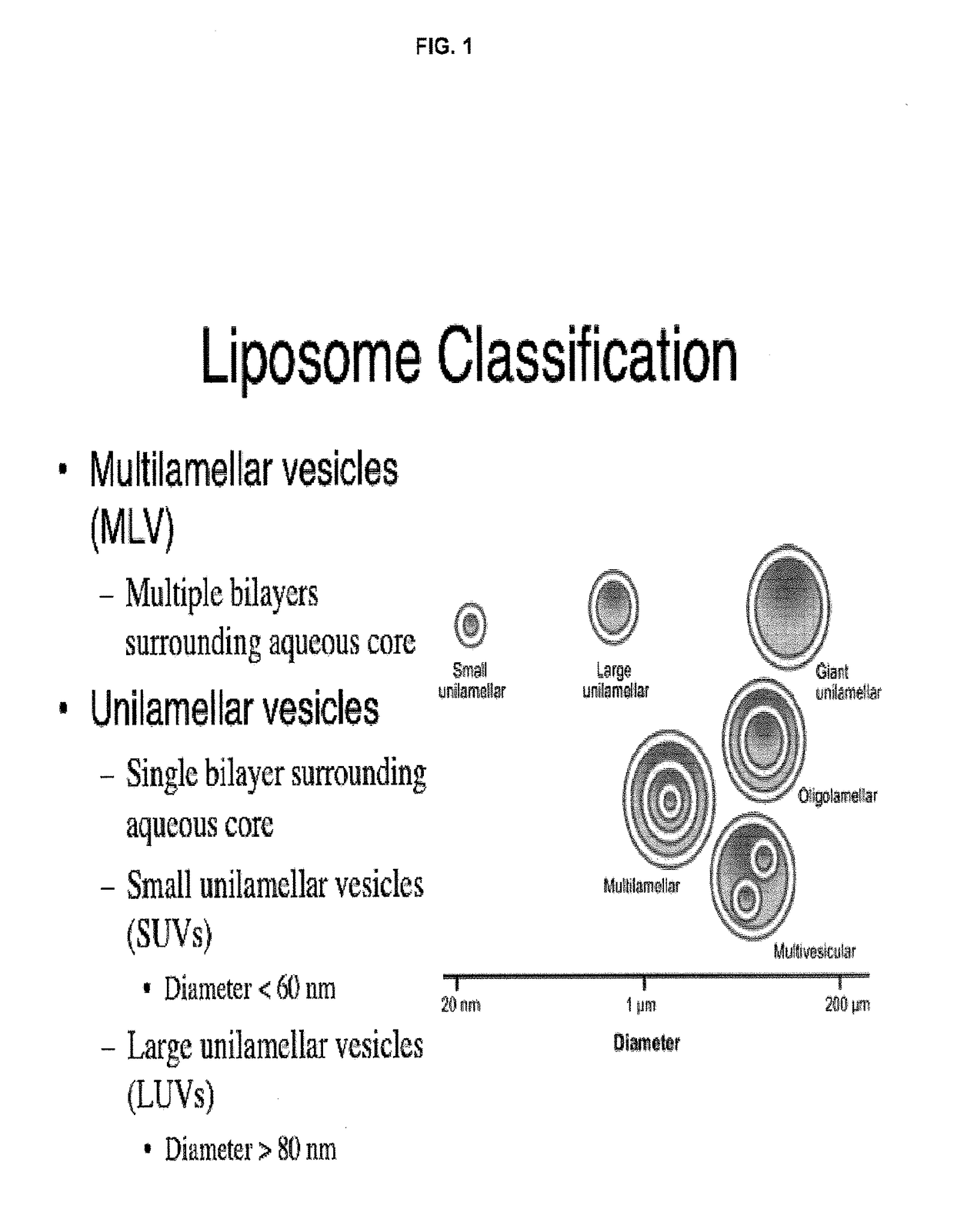
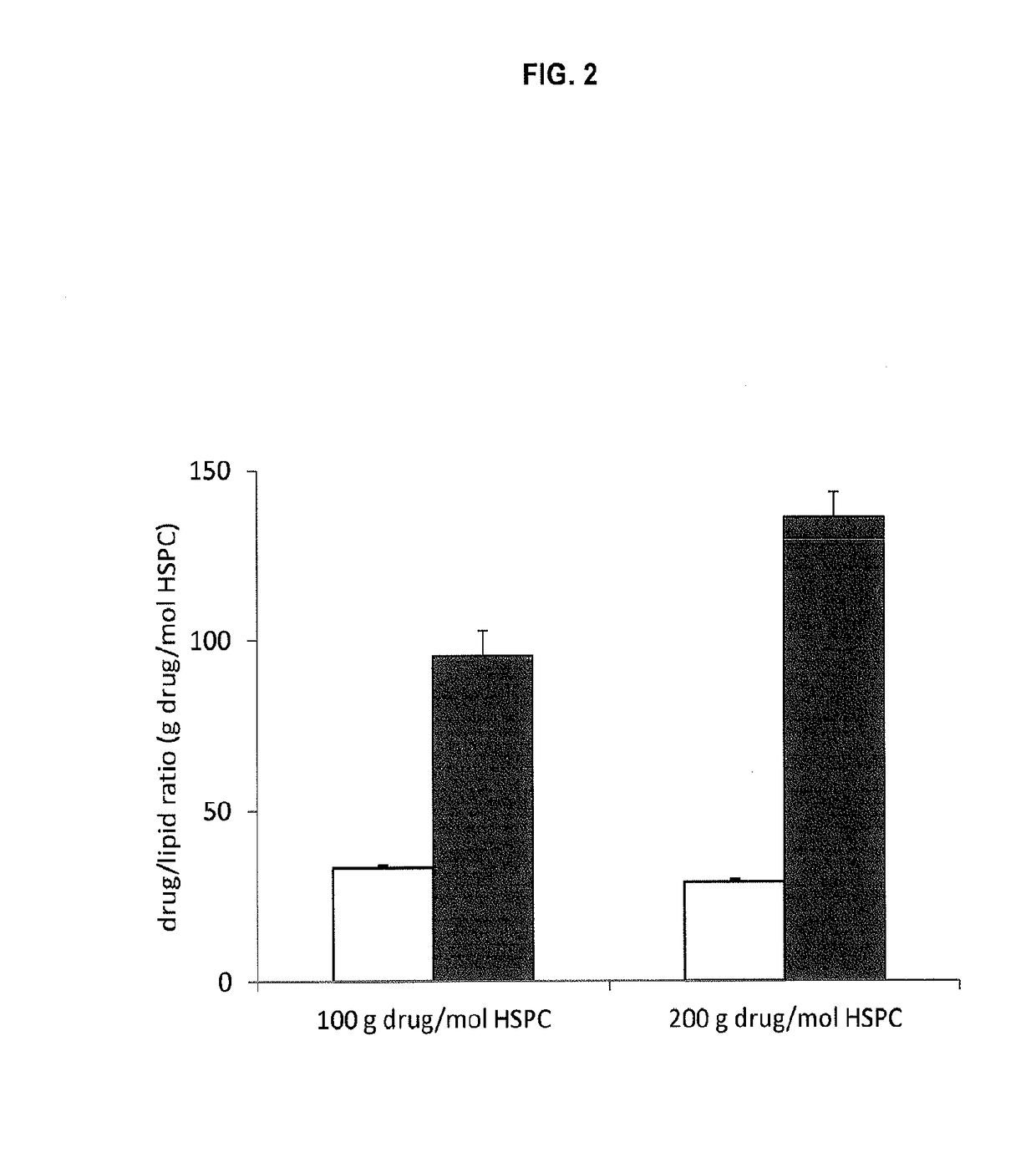
A method for encapsulation of antineoplastic agents in liposomes is provided, having preferably a high drug:lipid ratio. Liposomes may be made by a process that loads the drug by an active mechanism using a transmembrane ion gradient, preferably a transmembrane pH gradient. Using this technique, trapping efficiencies approach 100%, and liposomes may be loaded with drug immediately prior to use, eliminating stability problems related to drug retention in the liposomes. Drug:lipid ratios employed are about 3-80 fold higher than for traditional liposome preparations, and the release rate of the drug from the liposomes is reduced. An assay method to determine free antineoplastic agents in a liposome preparation is also disclosed.
Owner:ELAN PHARM INC
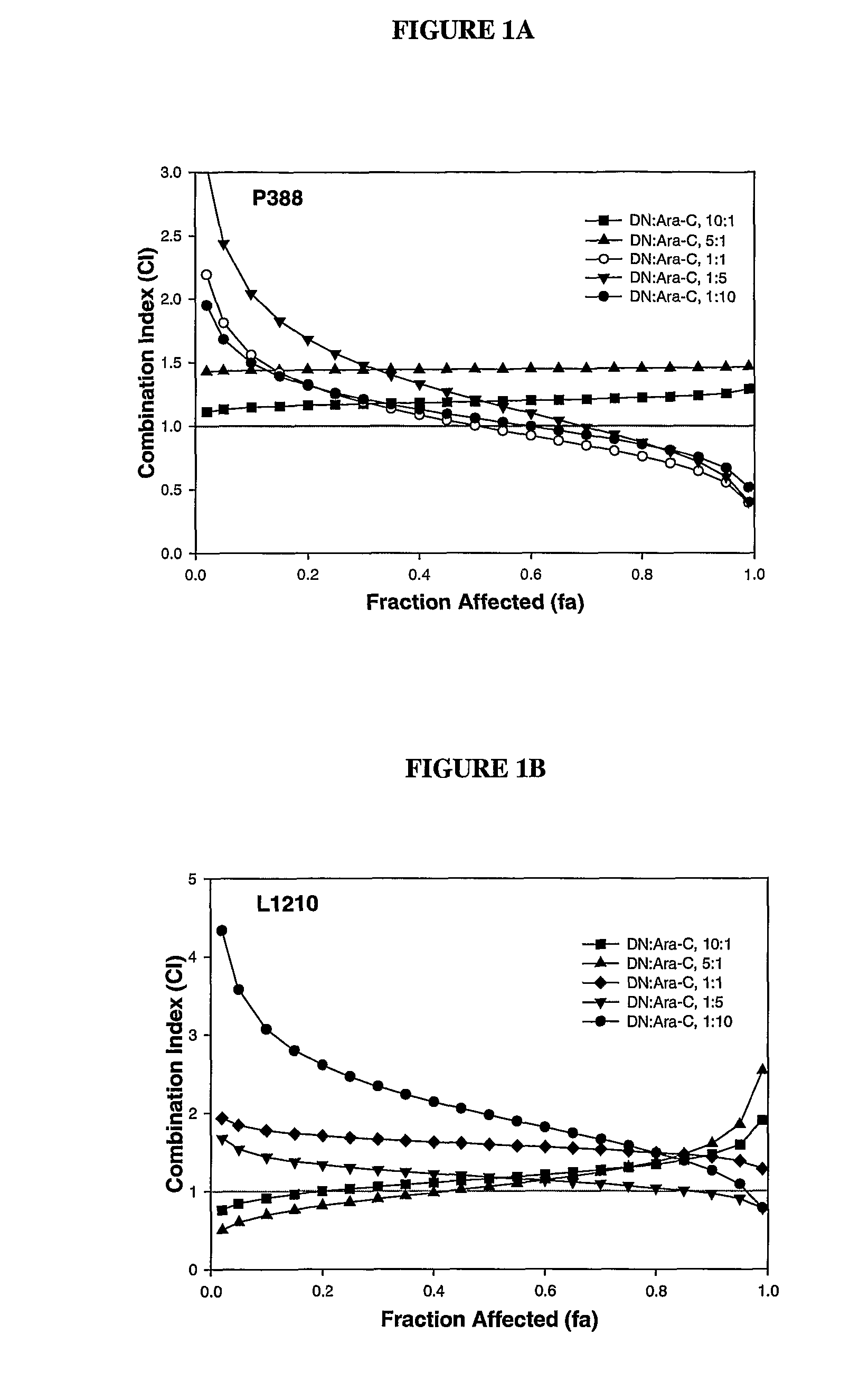
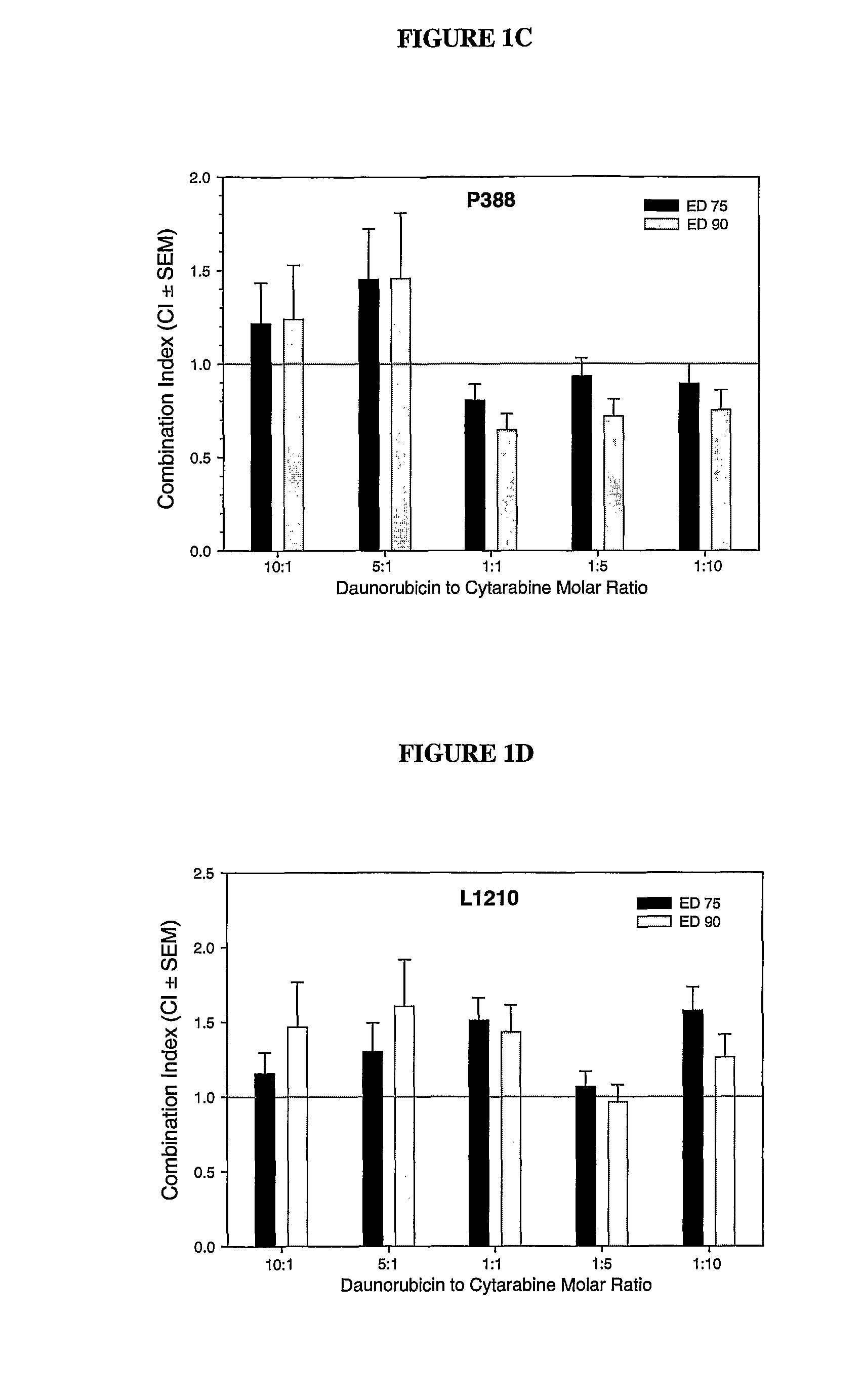
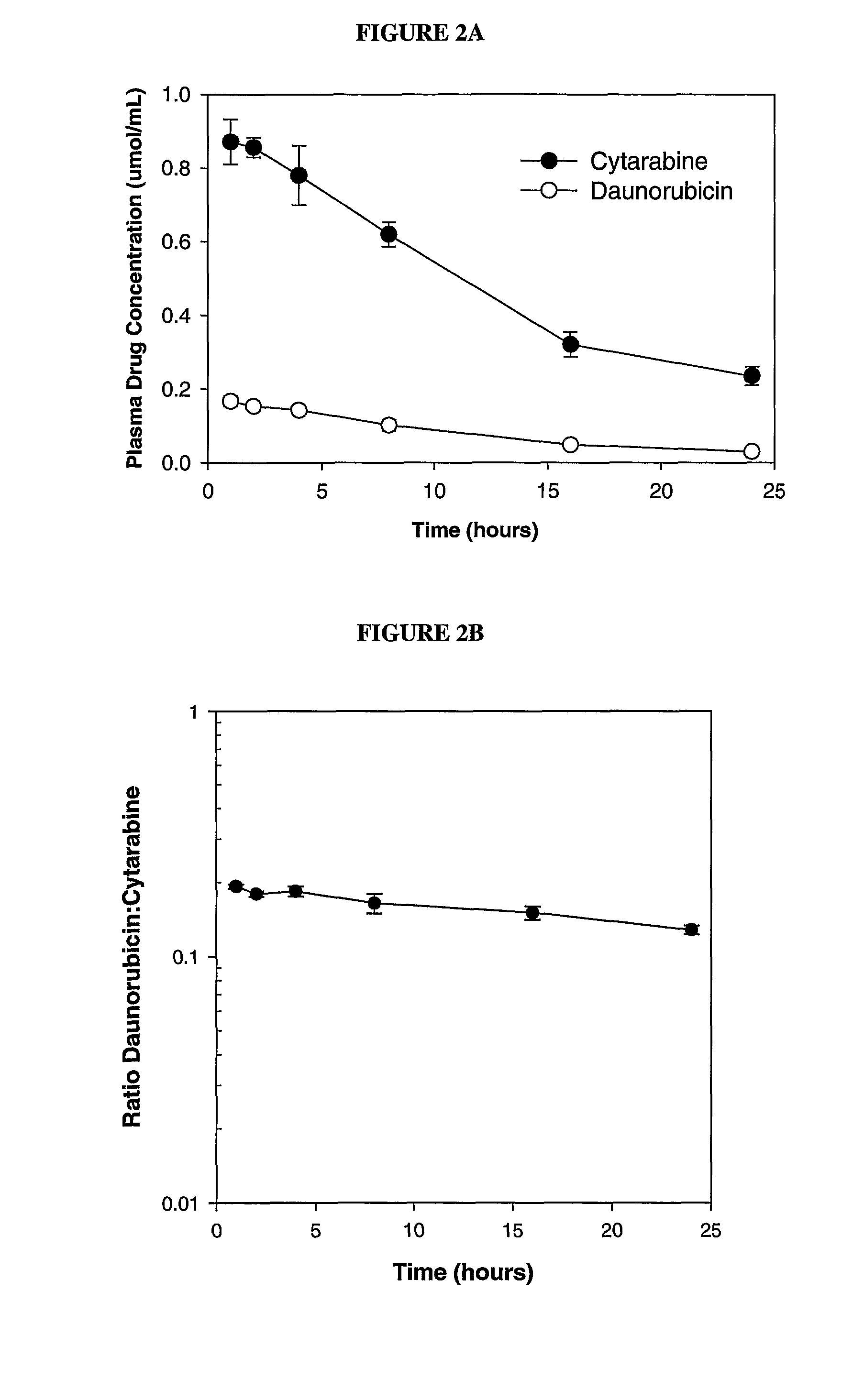
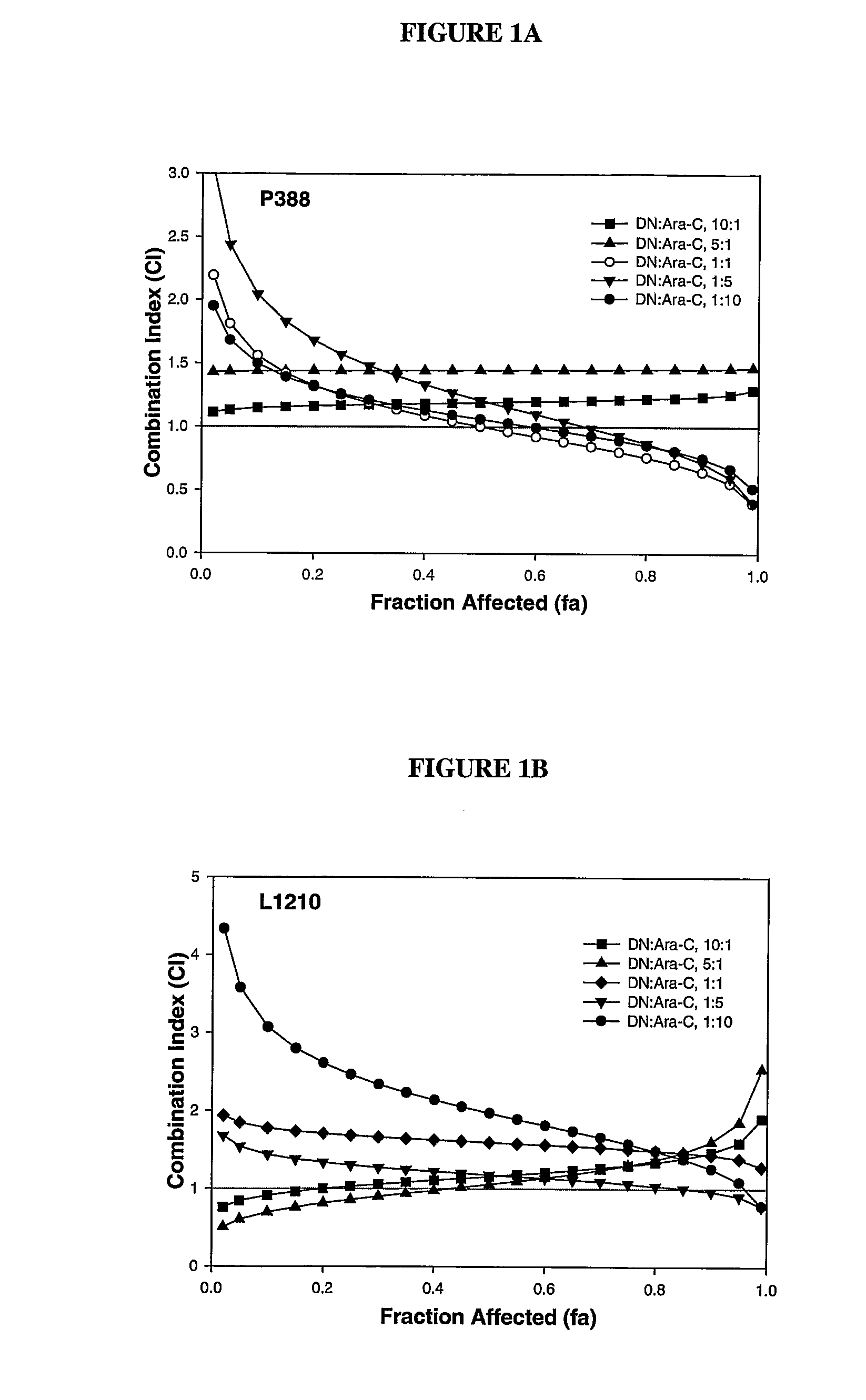
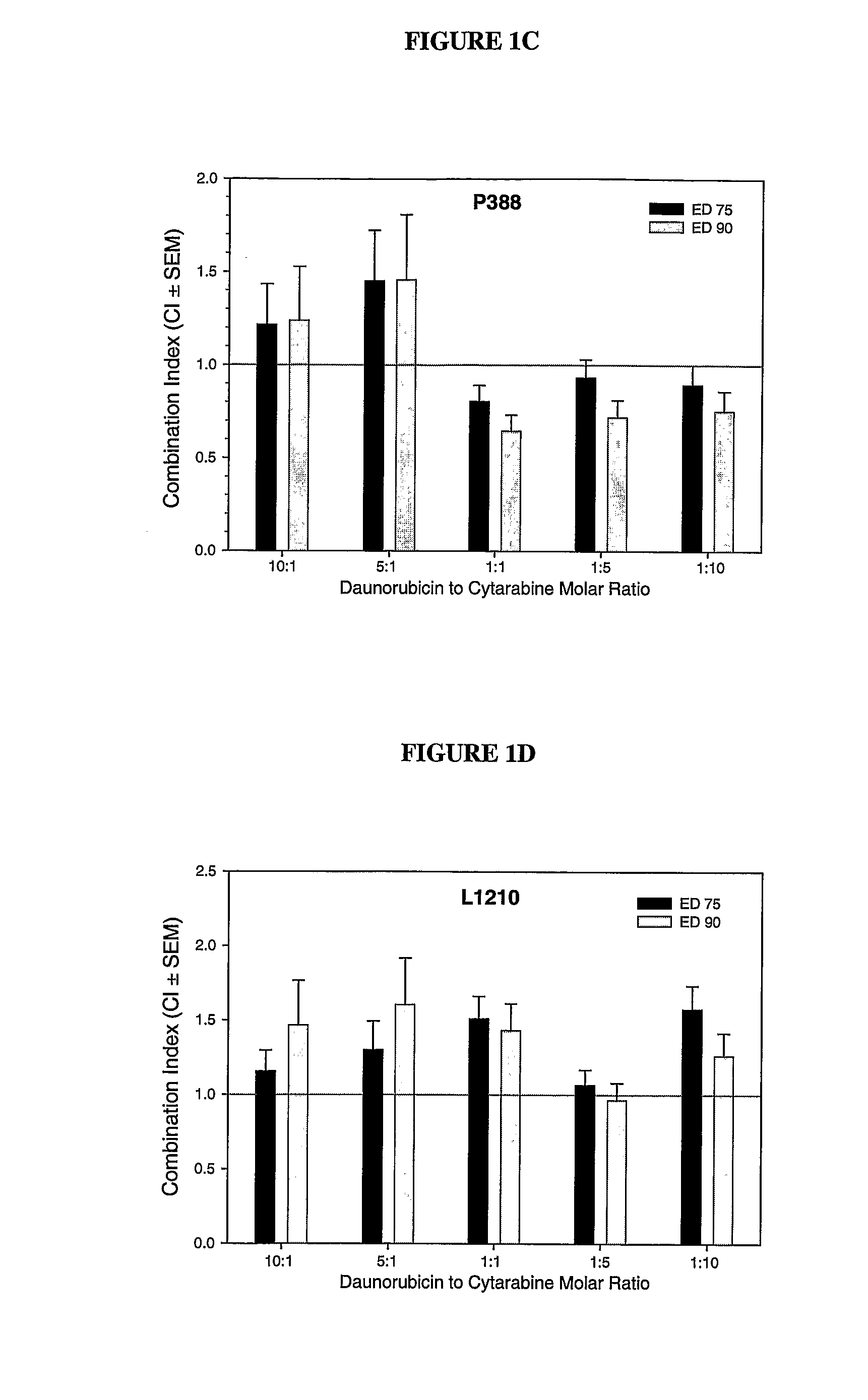
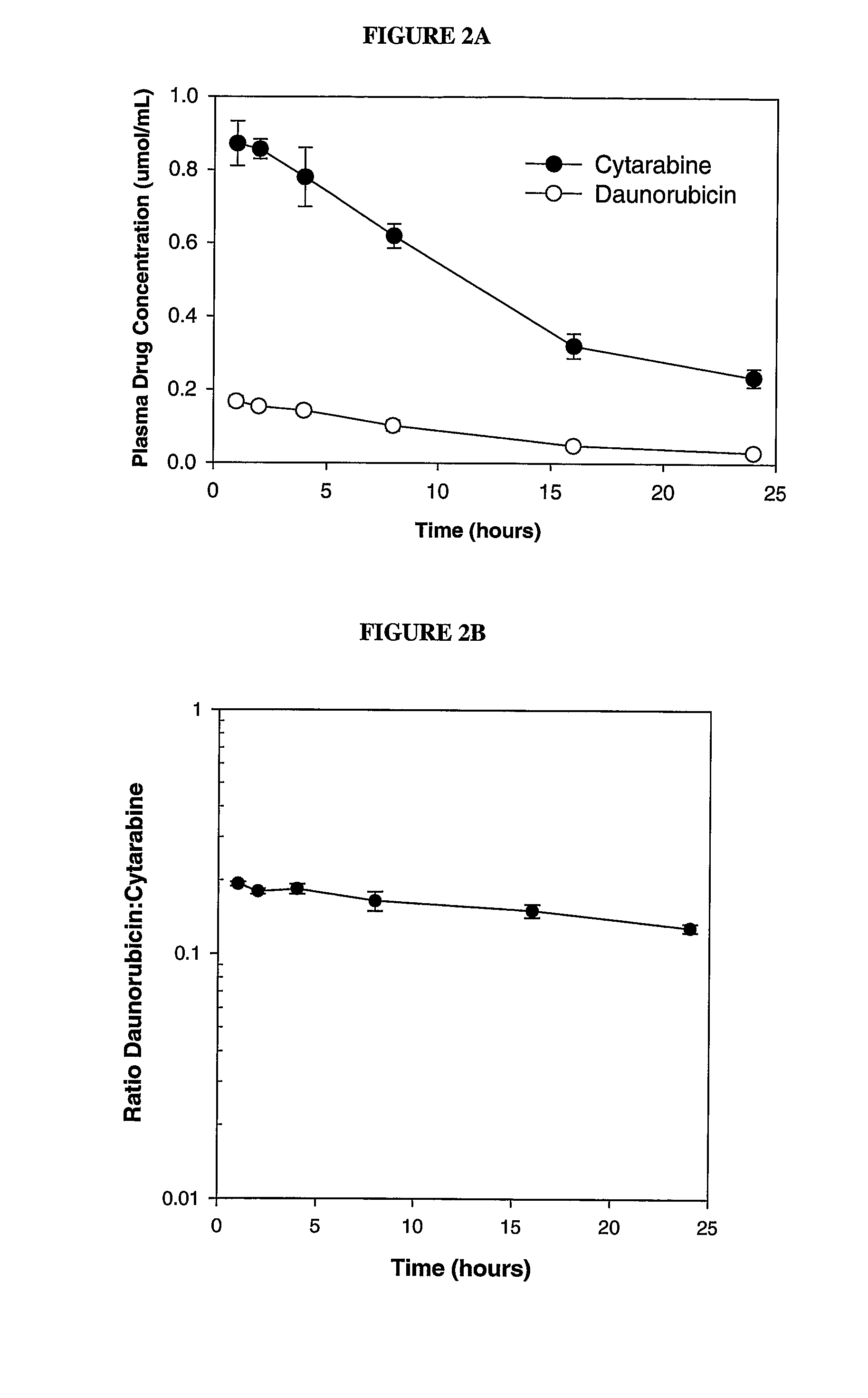
Liposomal formulations of anthracycline agents and cytidine analogs
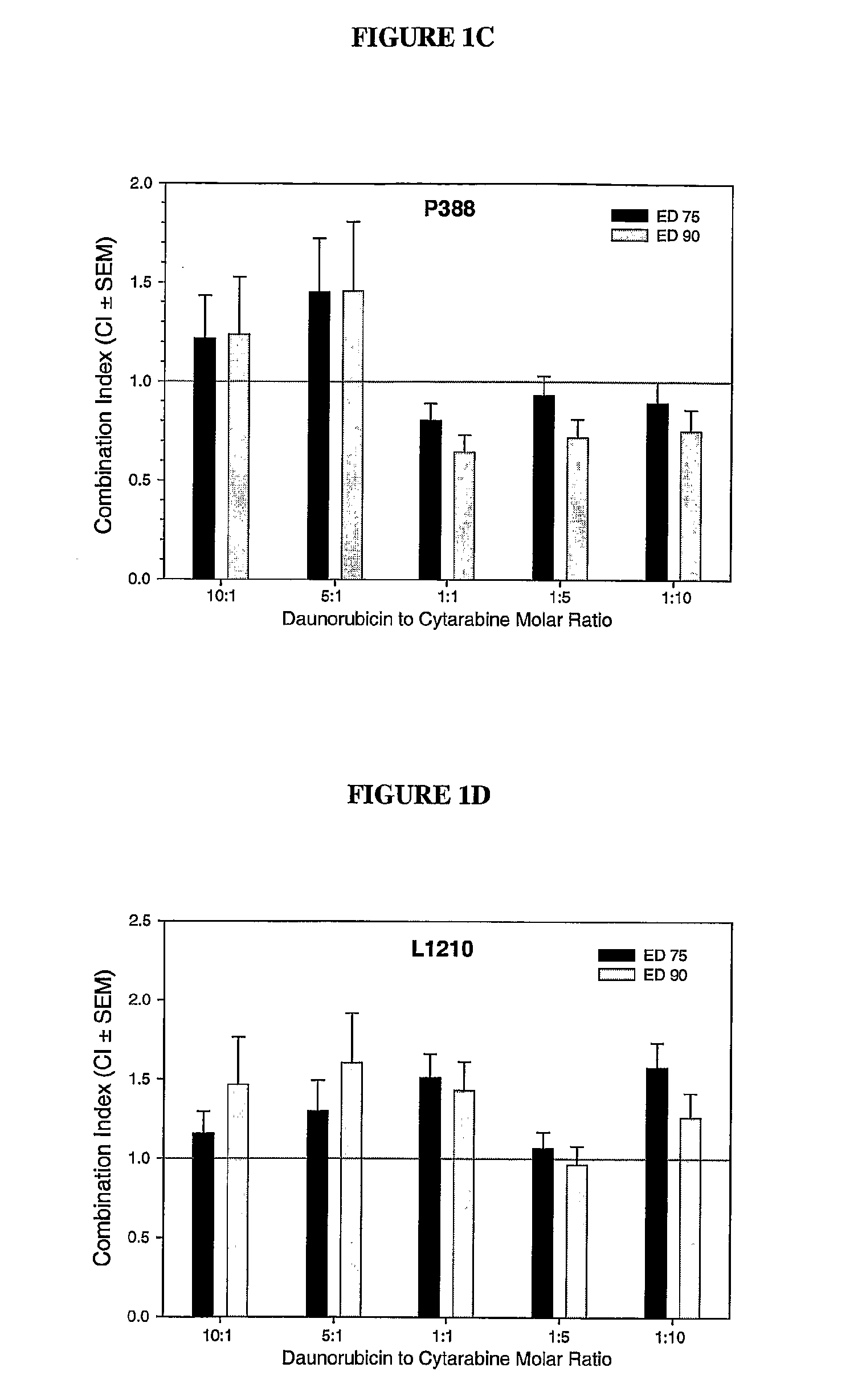
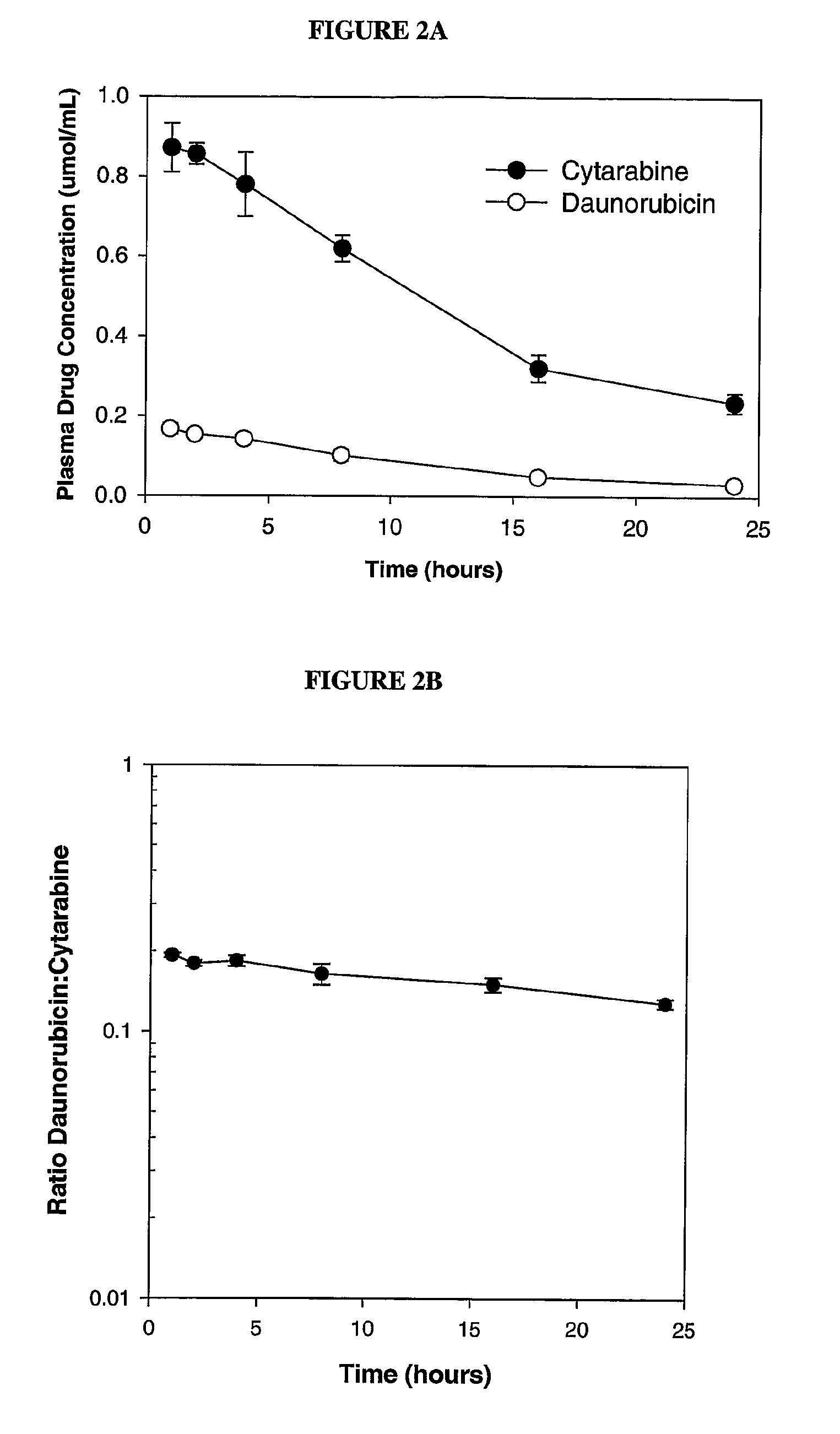
Compositions which comprise an anthracycline agent, and a cytidine analog are encapsulated in liposomal carriers. The preferred anthracycline agent is selected from the group of daunorubicin, doxorubicin, and idarubicin, while the preferred cytidine analog is selected from the group of cytarabine, gemcitabine, or 5-azacytidine. The combination of the anthracycline agent and cytidine analog encapsulated in said liposomal carriers are useful in achieving a drug retention and a sustained drug release for each therapeutic agent.
Owner:CELATOR PHARMA INC
Lipid carrier compositions and methods for improved drug retention
InactiveUS20050118249A1Increased systemic retentionIncrease contentLiposomal deliveryLipid formationCholesterol
Liposomal compositions which have enhanced retention properties for biological agents are characterized by an intrasomal osmolarity of 500 mOSM / kg or less and by containing substantially no cholesterol. The liposomes comprise vesicle forming lipids along with aggregation preventing components, and typically have transition temperatures of 38° C. or higher.
Owner:CELATOR PHARMA INC
Remote loading of sparingly water-soluble drugs into liposomes
InactiveUS20140220111A1Raise the ratioCost efficiencyOrganic active ingredientsInorganic non-active ingredientsLipid formationDisease
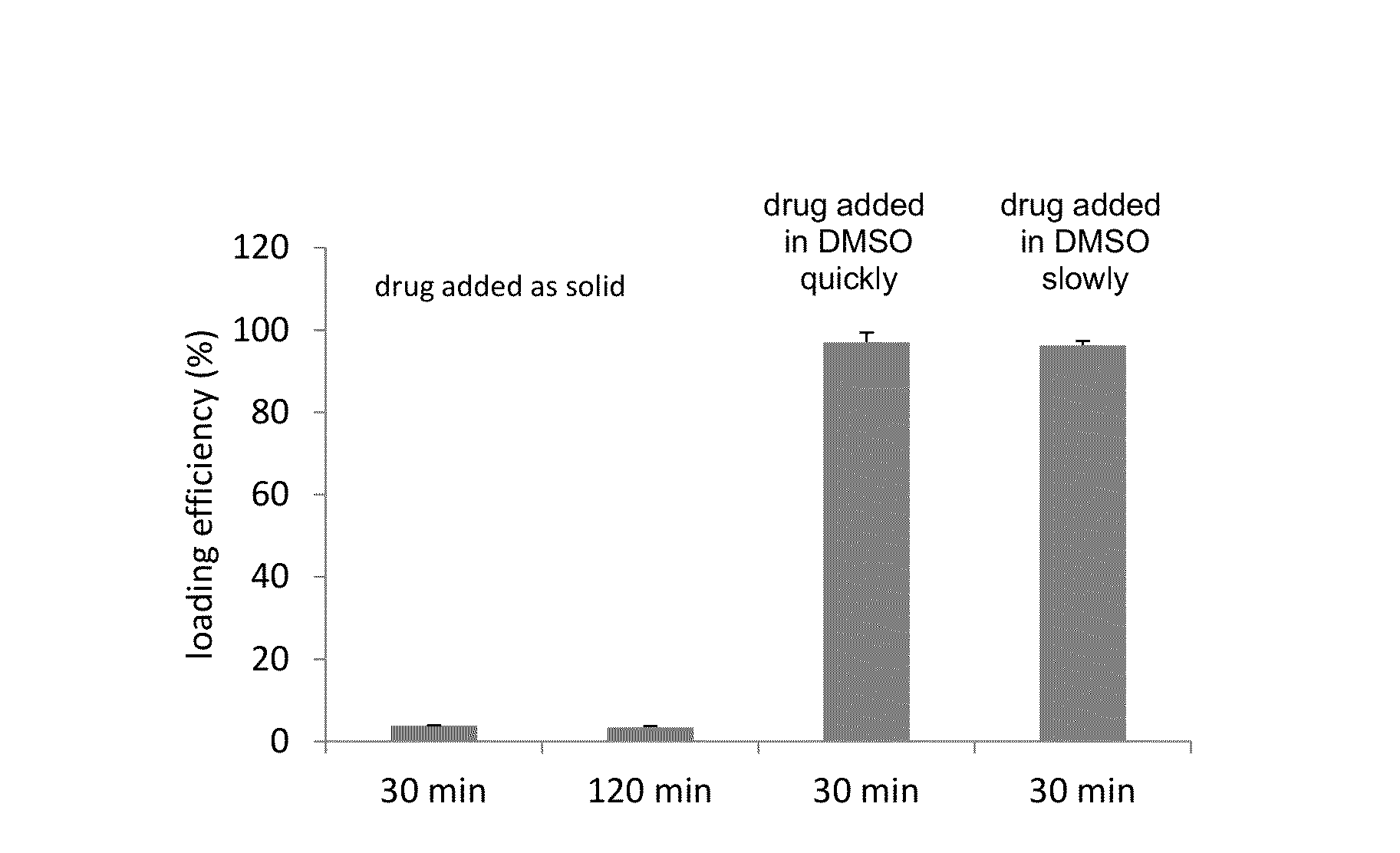
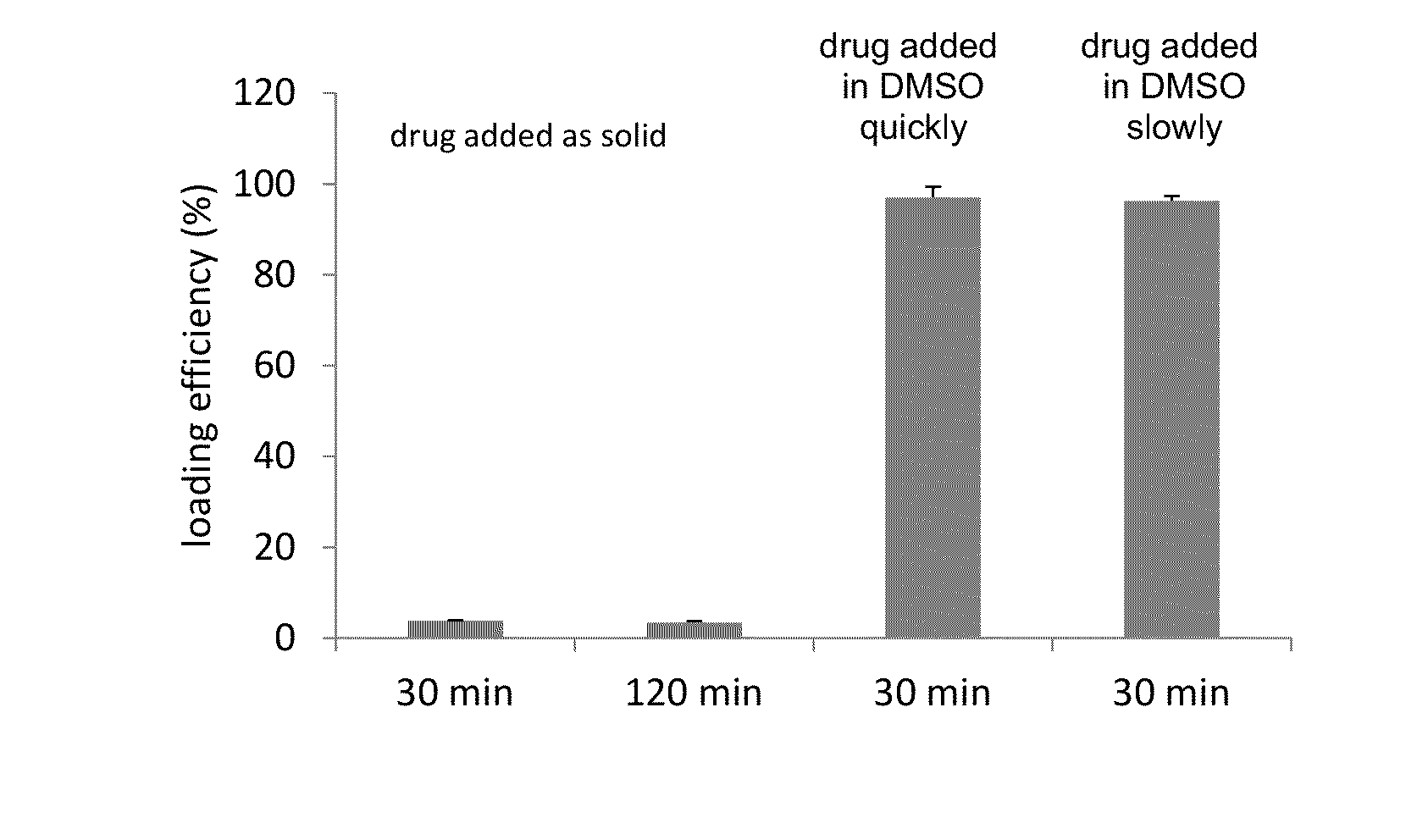
The present invention provides liposome compositions containing sparingly soluble drugs that are used to treat life-threatening diseases. A preferred method of encapsulating a drug inside a liposome is by remote or active loading. Remote loading of a drug into liposomes containing a transmembrane electrochemical gradient is initiated by co-mixing a liposome suspension with a solution of drug, whereby the neutral form of the compound freely enters the liposome and becomes electrostatically charged thereby preventing the reverse transfer out of the liposome. There is a continuous build-up of compound within the liposome interior until the electrochemical gradient is dissipated or all the drug is encapsulated in the liposome. However, this process as described in the literature has been limited to drugs that are freely soluble in aqueous solution or solubilized as a water-soluble complex. This invention describes compositions and methods for remote loading drugs with low water solubility (<2 mg / mL). In the preferred embodiment the drug in the solubilizing agent is mixed with the liposomes in aqueous suspension so that the concentration of solubilizing agent is lowered to below its capacity to completely solubilize the drug. This results in the drug precipitating but remote loading capability is retained. The process is scalable and, in liposomes in which the lipid composition and remote loading agent are optimized, the resulting drug-loaded liposomes are characterized by a high drug-to-lipid ratios and prolonged drug retention when the liposome encapsulated drug is administered to a subject.
Owner:ZONEONE PHARMA
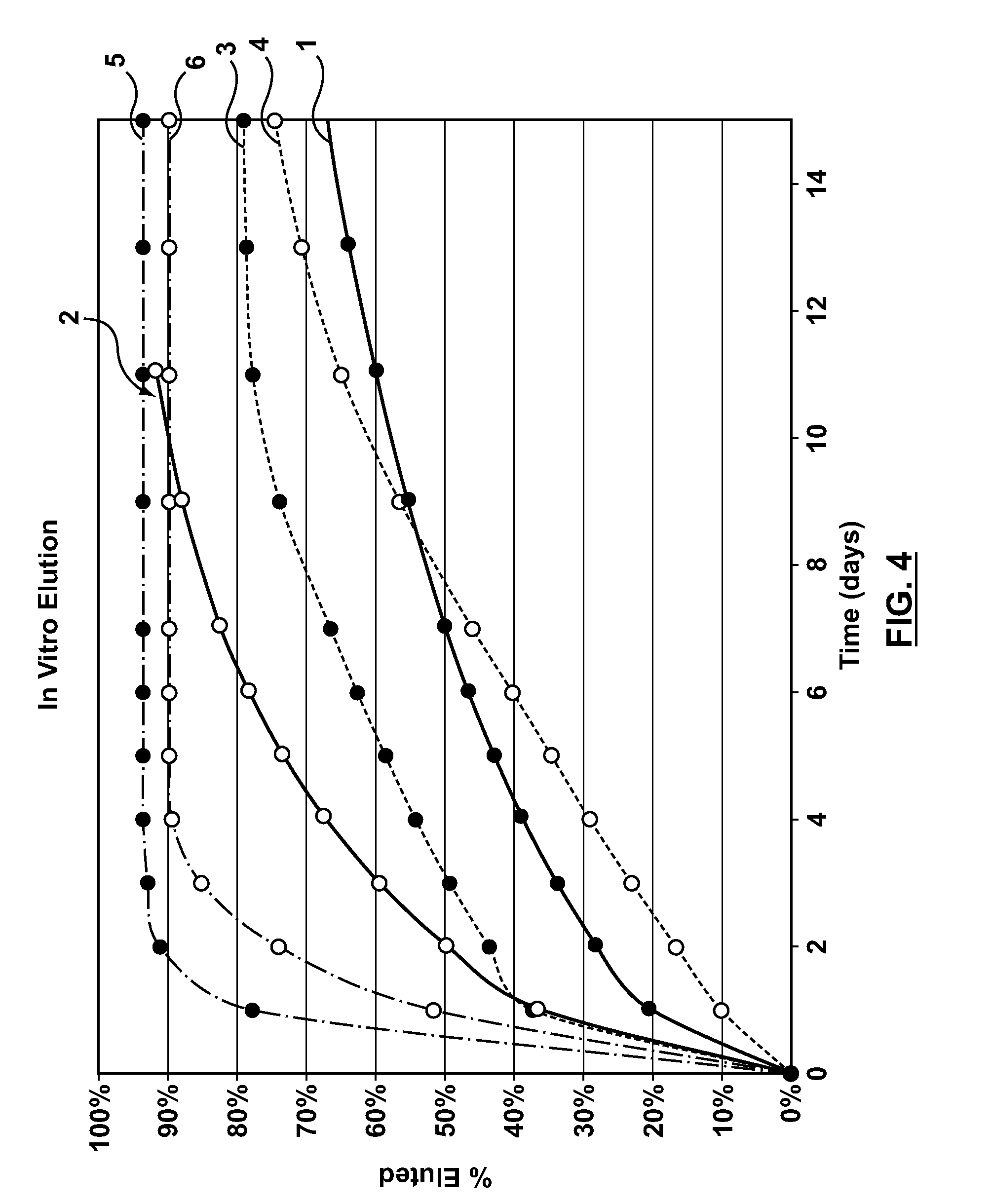
Coating system and method for drug elution management
ActiveUS20110086081A1Block mobilizationPromote retention of drugBiocidePeptide/protein ingredientsCoating systemPharmaceutical drug
The teachings are directed to a medical device having a drug-retaining coating that at least substantially delays the initial elution of a drug for a time effective at forming a functional endothelium over a surface of the medical device.
Owner:ALTAI MEDICAL TECH
Remote loading of sparingly water-soluble drugs into liposomes
InactiveUS20140220110A1Raise the ratioCost efficiencyBiocideInorganic non-active ingredientsDiseaseElectrochemical gradient
The present invention provides liposome compositions containing sparingly soluble drugs that are used to treat life-threatening diseases. A preferred method of encapsulating a drug inside a liposome is by remote or active loading. Remote loading of a drug into liposomes containing a transmembrane electrochemical gradient is initiated by co-mixing a liposome suspension with a solution of drug, whereby the neutral form of the compound freely enters the liposome and becomes electrostatically charged thereby preventing the reverse transfer out of the liposome. There is a continuous build-up of compound within the liposome interior until the electrochemical gradient is dissipated or all the drug is encapsulated in the liposome. However, this process as described in the literature has been limited to drugs that are freely soluble in aqueous solution or solubilized as a water-soluble complex. This invention describes compositions and methods for remote loading drugs with low water solubility (<2 mg / mL). In the preferred embodiment the drug in the solubilizing agent is mixed with the liposomes in aqueous suspension so that the concentration of solubilizing agent is lowered to below its capacity to completely solubilize the drug. This results in the drug precipitating but remote loading capability is retained. The process is scalable and, in liposomes in which the lipid composition and remote loading agent are optimized, the resulting drug-loaded liposomes are characterized by a high drug-to-lipid ratios and prolonged drug retention when the liposome encapsulated drug is administered to a subject.
Owner:ZONEONE PHARMA
Liposome compositions for improved drugretention
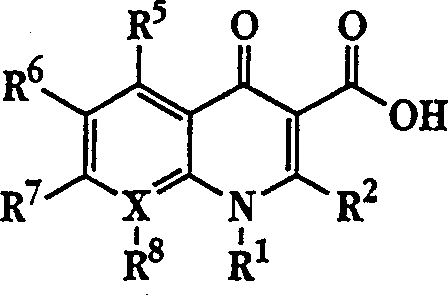
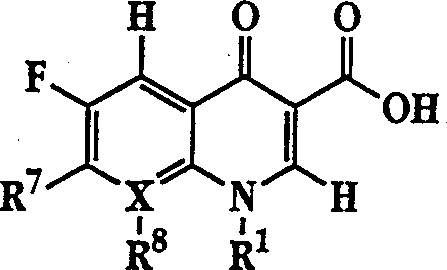
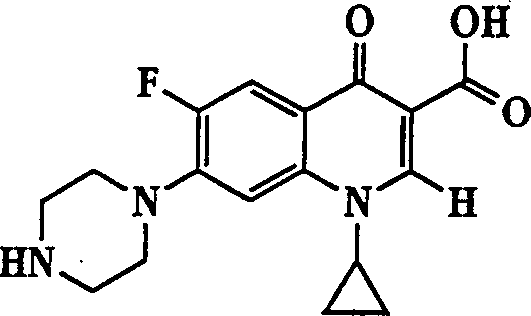
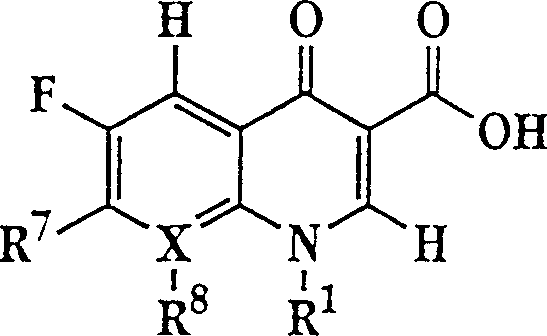
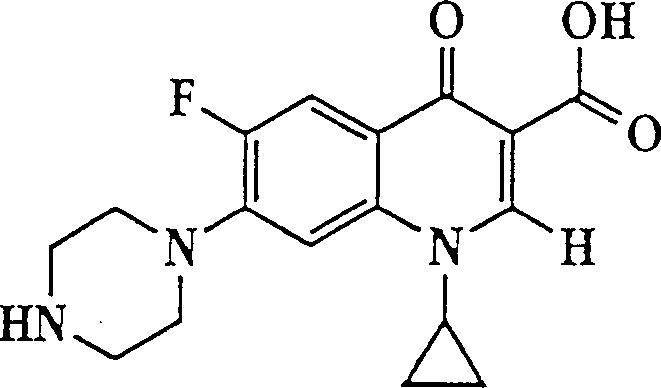
The invention describes a liposome composition for enhanced retention of a quinolone compound, and in particular of a fluoroquinolone compound. Entrapped in the liposomes is a polyanionic polymer effective to form a complex with the fluoroquinolone. A method of preparing the liposomes which includes a process for removal of unentrapped polyanionic polymer is also described.
Owner:ALZA CORP
Apparatus and methods for loading a drug eluting medical device
Methods and apparatus are disclosed for loading a therapeutic substance or drug within a lumenal space of a hollow wire having a plurality of side openings along a length thereof that forms a hollow drug-eluting stent with a plurality of side drug delivery openings. Loading a drug within the lumenal space of the hollow stent includes a drug filling step, in which the drug is mixed with a solvent or dispersion medium. The lumenal space may be filled with the drug solution or suspension in a reverse fill process and / or a forward fill process. After the drug filling step, a solvent or dispersion medium extracting step is performed to extract the solvent or dispersion medium from within the lumenal space such that only the drug remains within the hollow stent. A stent cleaning step may be performed to an exterior surface of the hollow stent.
Owner:MEDTRONIC VASCULAR INC
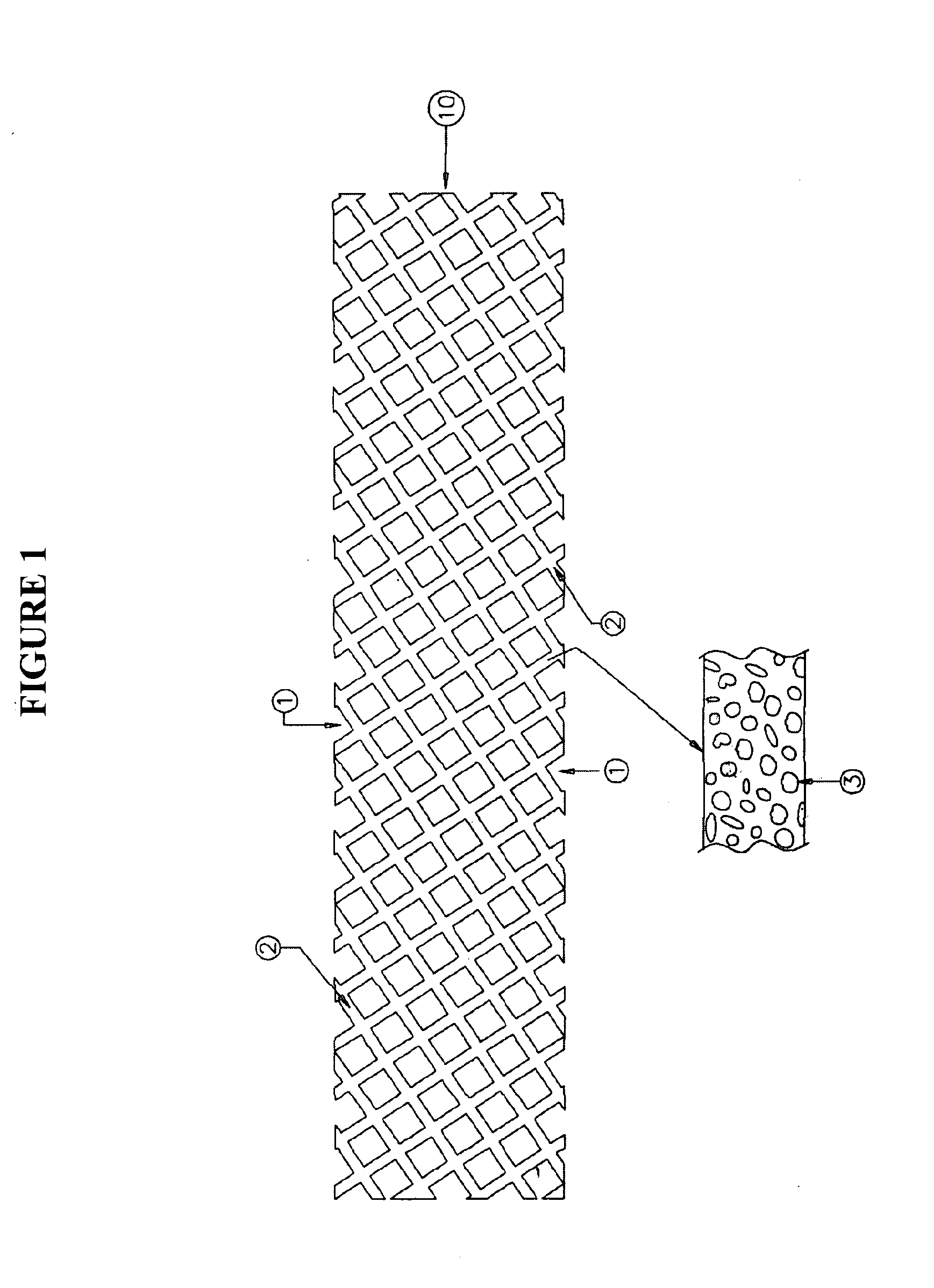
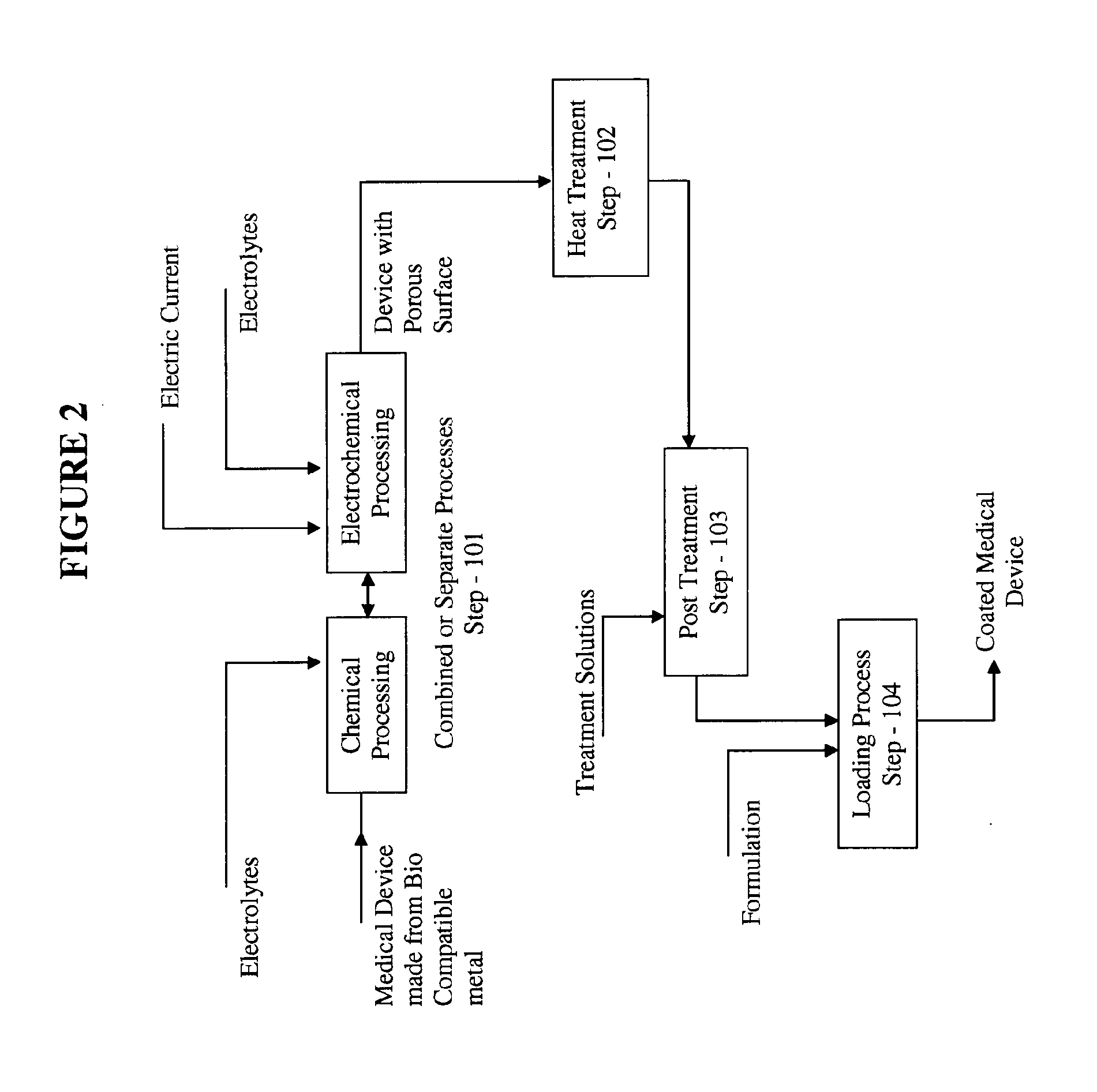
Medical device loaded with formulation for targeted delivery of biologically active material/s and method of manufacture thereof
InactiveUS20110274732A1High and nearly complete drug availabilityEasy to keepBiocideStentsPercent Diameter StenosisDrug release
The present invention relates to targeted drug delivery of a drug or therapeutic agent through medical devices coated with formulations comprising of therapeutic agent. The coating on medical devices comprises of therapeutic agent(s), affinity vehicle(s) and additives for targeted drug delivery of biologically active material(s). The invention provides a method of manufacturing the formulation, method of coating the medical devices with such formulations to achieve controlled delivery of optimum drug dose at the target site within the body, desirable drug retention on the medical devices in vivo and in vitro and desirable drug release at the target tissue in-vivo. The invention this provides a mechanism to enhance the bioavailability of the therapeutic agent at the target tissue in the treatment of restenosis thereby reduces the actual dose of the therapeutic agent and provides a very thin layer of coating on the surface of the medical device.
Owner:MERIL LIFE SCI PVT
Liposomal Formulations of Anthracycline Agents and Cytidine Analogs
Compositions which comprise an anthracycline agent, and a cytidine analog are encapsulated in liposomal carriers. The preferred anthracycline agent is selected from the group of daunorubicin, doxorubicin, and idarubicin, while the preferred cytidine analog is selected from the group of cytarabine, gemcitabine, or 5-azacytidine. The combination of the anthracycline agent and cytidine analog encapsulated in said liposomal carriers are useful in achieving a drug retention and a sustained drug release for each therapeutic agent.
Owner:CELATOR PHARMA INC
Remote loading of sparingly water-soluble drugs into liposomes
ActiveUS20160324780A1Raise the ratioCost efficiencyOrganic active ingredientsInorganic non-active ingredientsDiseaseElectrochemical gradient
The present invention provides liposome compositions containing sparingly soluble drugs that are used to treat life-threatening diseases. A preferred method of encapsulating a drug inside a liposome is by remote or active loading. Remote loading of a drug into liposomes containing a transmembrane electrochemical gradient is initiated by co-mixing a liposome suspension with a solution of drug, whereby the neutral form of the compound freely enters the liposome and becomes electrostatically charged thereby preventing the reverse transfer out of the liposome. There is a continuous build-up of compound within the liposome interior until the electrochemical gradient is dissipated or all the drug is encapsulated in the liposome. However, this process as described in the literature has been limited to drugs that are freely soluble in aqueous solution or solubilized as a water-soluble complex. This invention describes compositions and methods for remote loading drugs with low water solubility (<2 mg / mL). In the preferred embodiment the drug in the solubilizing agent is mixed with the liposomes in aqueous suspension so that the concentration of solubilizing agent is lowered to below its capacity to completely solubilize the drug. This results in the drug precipitating but remote loading capability is retained. The process is scalable and, in liposomes in which the lipid composition and remote loading agent are optimized, the resulting drug-loaded liposomes are characterized by a high drug-to-lipid ratios and prolonged drug retention when the liposome encapsulated drug is administered to a subject.
Owner:CELATOR PHARMA INC
Immunosupportive drug sparing diet
InactiveUS6706691B1Enhance immunosuppressive potencyHigh expressionBiocideMicrobiological testing/measurementIsozymeBioavailability
The present invention is directed to immunosupportive, drug sparing diets and methods of using these diets. The compositions and methods disclosed herein increase the bioavailability of pharmaceutical compositions metabolized by the gut P450 isozymes.
Owner:RICE UNIV +1
Remote loading of sparingly water-soluble drugs into lipid vesicles
ActiveUS20170224715A1Cost efficiencyReduce the amount requiredPowder deliveryOrganic active ingredientsLipid formationDisease
The present invention provides liposome compositions containing sparingly soluble drugs that are used to treat life-threatening diseases. A preferred method of encapsulating a drug inside a liposome is by remote or active loading. However, this process as described in the literature has been limited to drugs that are freely soluble in aqueous solution or solubilized as a water-soluble complex. This invention describes compositions and methods for remote loading drugs with low water solubility (<2 mg / mL). In the preferred embodiment the drug in the solubilizing agent is mixed with the liposomes in aqueous suspension so that the concentration of solubilizing agent is lowered to below its capacity to completely solubilize the drug. This results in the drug precipitating but remote loading is capability retained. The process is scalable and the resulting drug-loaded liposomes are characterized by a high drug-to-lipid ratios and predictable drug retention when the liposome encapsulated drug is administered in patients.
Owner:CELATOR PHARMA INC
Micelle composition of polymer and passenger drug
Hydrophobic drugs become more practical for treatments by being encapsulated in micelle compositions for increasing solubility. Micelle compositions may include an excipient tocopherol and / or prodrug formulations of the drug. Micelles extend the time period the drug remains in the micelles to improve drug circulation time and thereby drug delivery. Hydrophobic drugs for micelle encapsulation may include rapamycin, geldanamycin, and paclitaxel. Administration of these micelle compositions does not require Cremophor EL or Tween 80, avoiding serious side effects associated with these products which would previously accompany such drug administration.
Owner:WISCONSIN ALUMNI RES FOUND
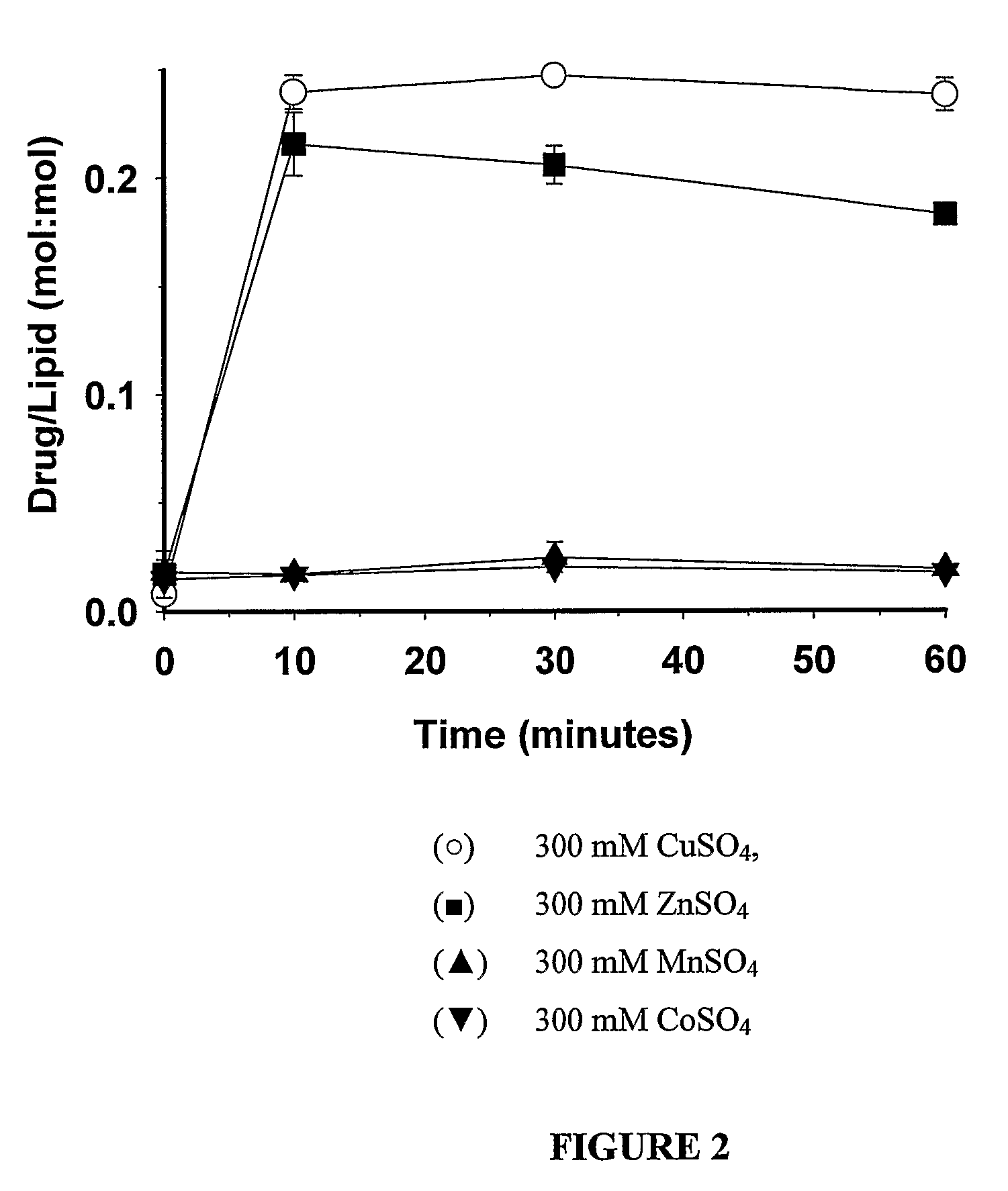
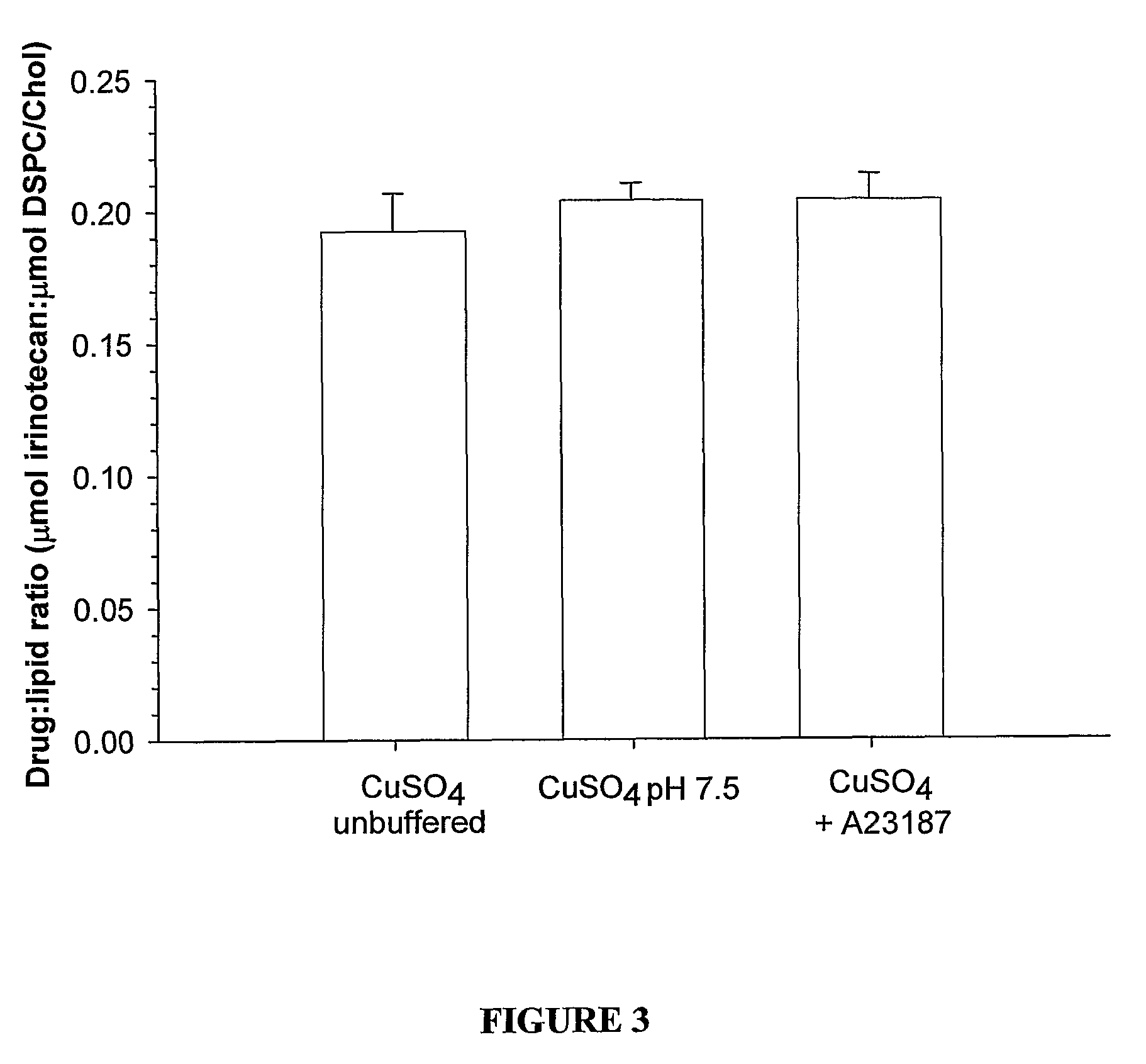
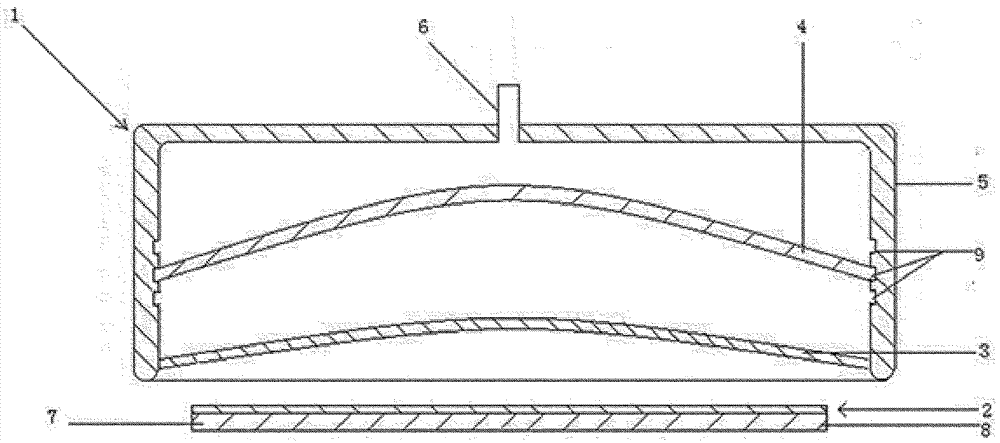
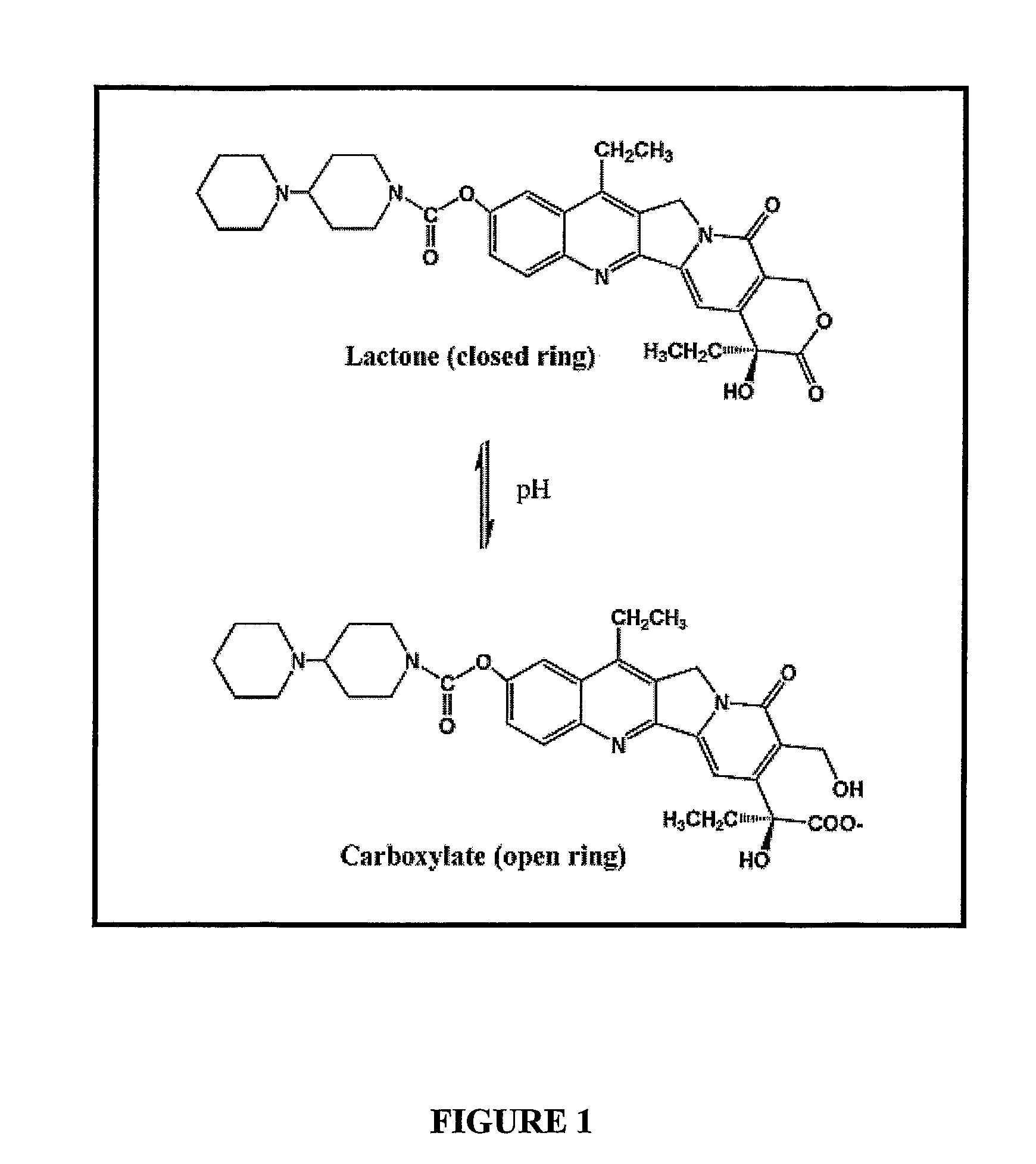
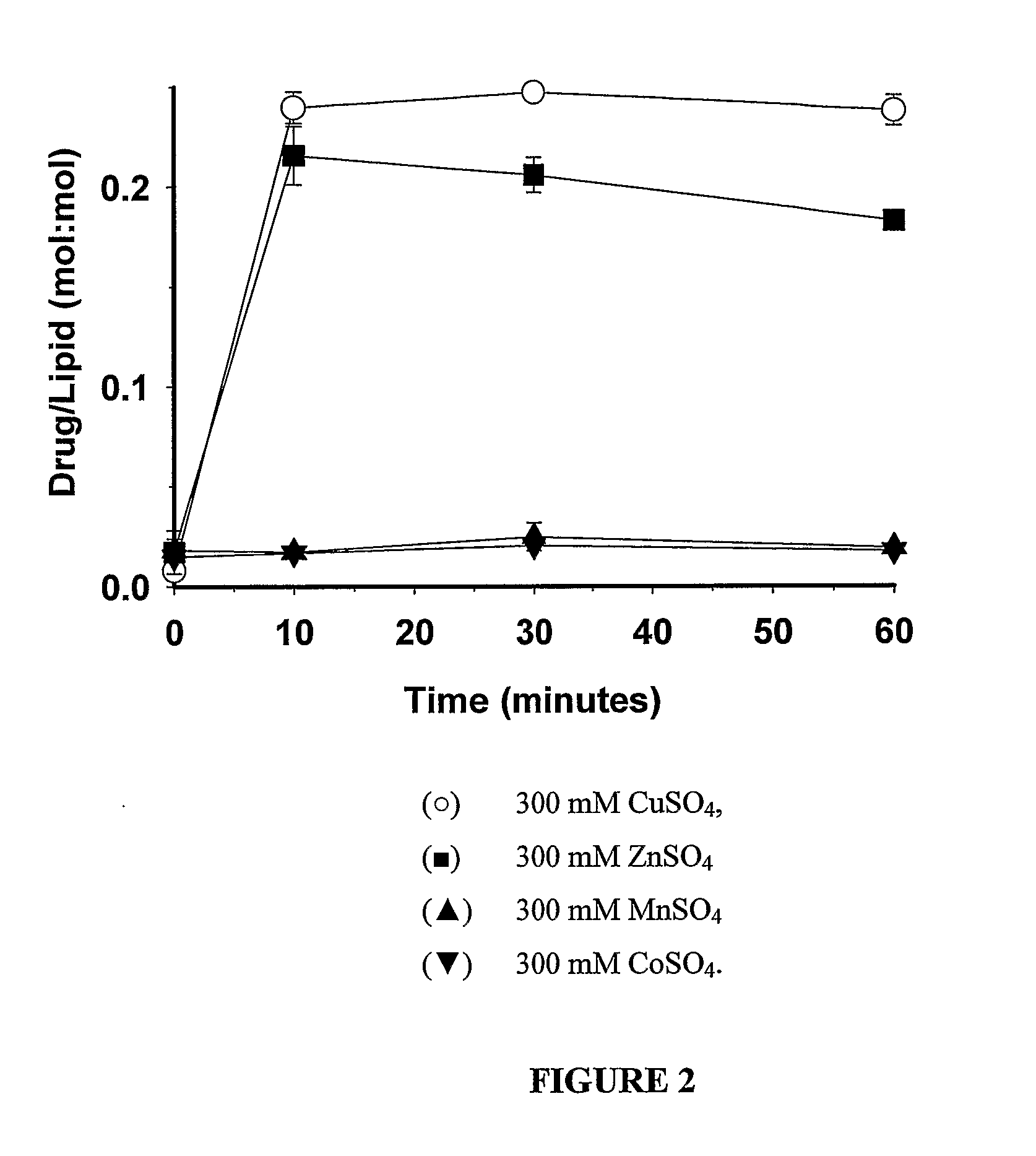
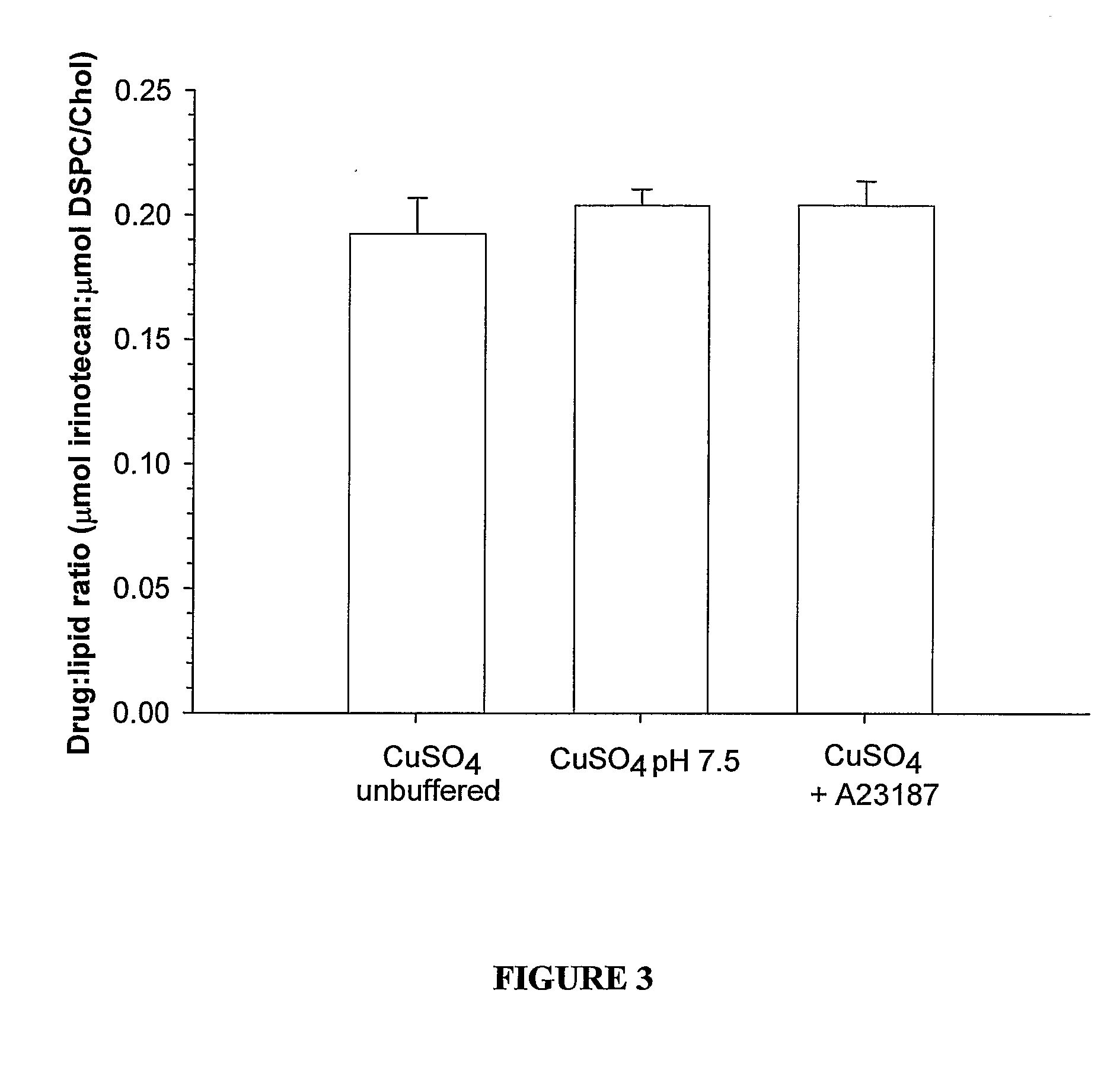
Liposomes with improved drug retention for treatment of cancer
InactiveUS8349360B2High retention rateOrganic active ingredientsInorganic non-active ingredientsIn vivoLiposome
The present invention relates to the use of copper ions to achieve enhanced retention of a therapeutic agent within a liposome. The invention may be employed to more effectively deliver a liposomally encapsulated therapeutic agent to a target site in vitro and in vivo for anti-cancer or other therapy. The liposome may comprise an interior buffer solution containing the therapeutic agent, the solution having a pH less than 6.5 and most preferably approximating pH 3.5. At least some of the copper ions are retained within the interior solution. In a particular embodiment the therapeutic agent may be a chemotherapeutic drug, such as irinotecan. The invention may also comprise an ionophore to facilitate loading of drug into the liposome. In one particular embodiment the combination of the ionophore A23187 and encapsulated divalent copper (Cu2+) resulted in an irinotecan formulation that exhibited surprisingly improved drug retention attributes.
Owner:BRITISH COLUMBIA CANCER AGENCY BRANCH
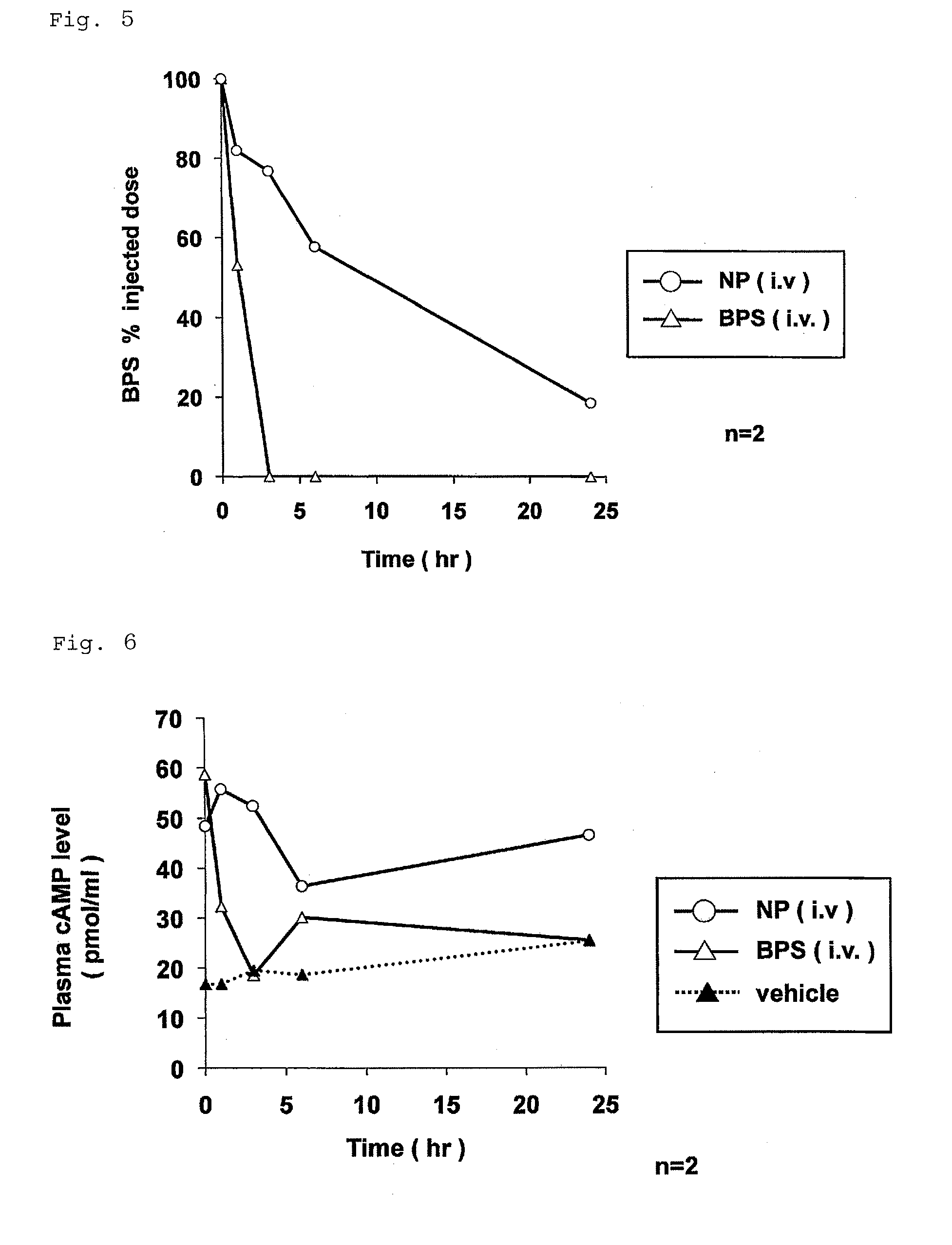
Nanoparticle containing prostaglandin I2 derivative
ActiveUS9161986B2Excels sustained releaseExcellent drug retentionPowder deliveryOrganic active ingredientsSide effectPolyethylene glycol
Owner:LTT BIO PHARMA
Remote loading of sparingly water-soluble drugs into liposomes
InactiveUS20170202776A1Raise the ratioCost efficiencyOrganic active ingredientsInorganic non-active ingredientsDrugs solutionDisease
The present invention provides liposome compositions containing sparingly soluble drugs that are used to treat life-threatening diseases. A preferred method of encapsulating a drug inside a liposome is by remote or active loading. Remote loading of a drug into liposomes containing a transmembrane electrochemical gradient is initiated by co-mixing a liposome suspension with a solution of drug, whereby the neutral form of the compound freely enters the liposome and becomes electrostatically charged thereby preventing the reverse transfer out of the liposome. There is a continuous build-up of compound within the liposome interior until the electrochemical gradient is dissipated or all the drug is encapsulated in the liposome. However, this process as described in the literature has been limited to drugs that are freely soluble in aqueous solution or solubilized as a water-soluble complex. This invention describes compositions and methods for remote loading drugs with low water solubility (<2 mg / mL). In the preferred embodiment the drug in the solubilizing agent is mixed with the liposomes in aqueous suspension so that the concentration of solubilizing agent is lowered to below its capacity to completely solubilize the drug. This results in the drug precipitating but remote loading capability is retained. The process is scalable and, in liposomes in which the lipid composition and remote loading agent are optimized, the resulting drug-loaded liposomes are characterized by a high drug-to-lipid ratios and prolonged drug retention when the liposome encapsulated drug is administered to a subject.
Owner:ZONEONE PHARMA
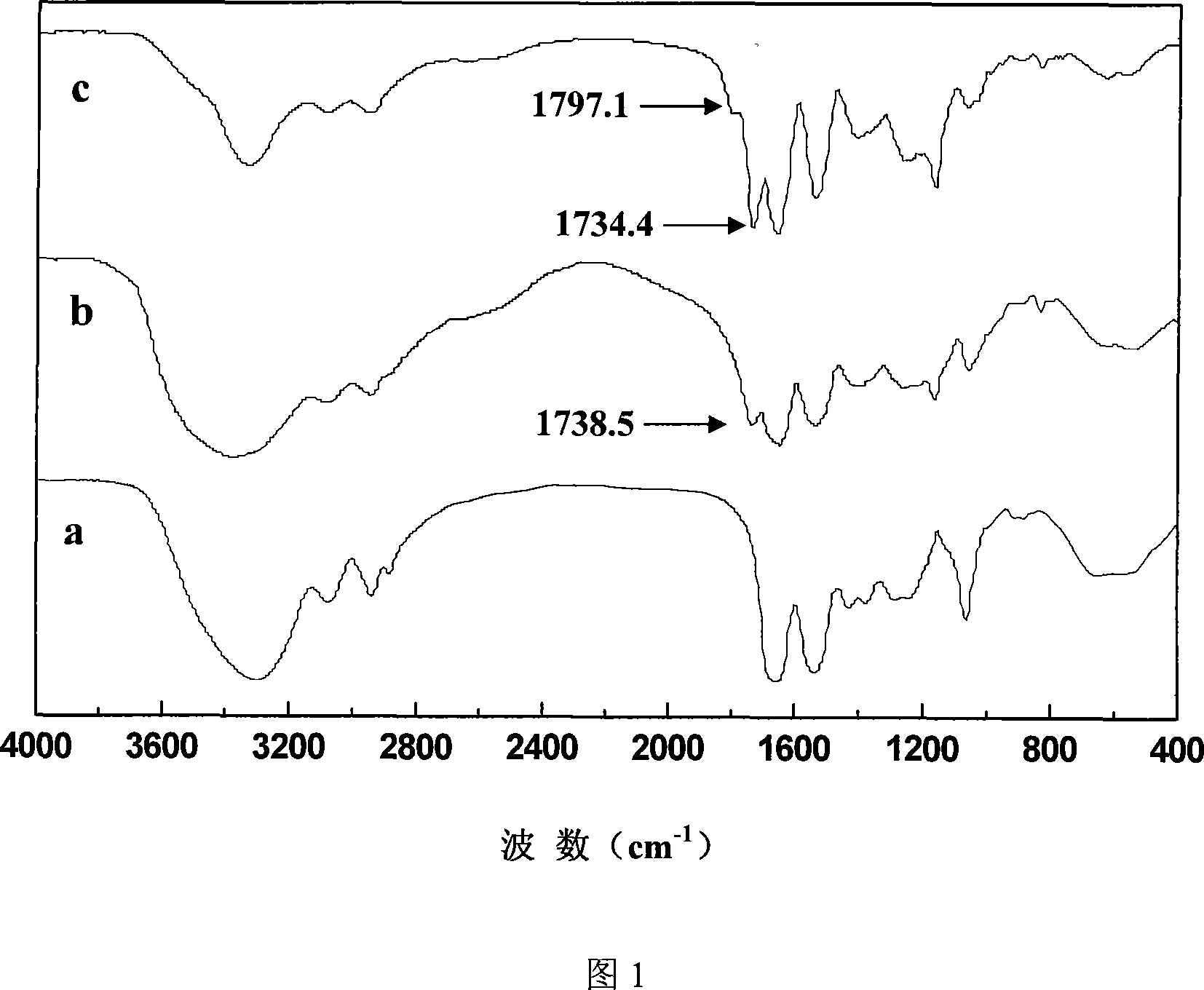
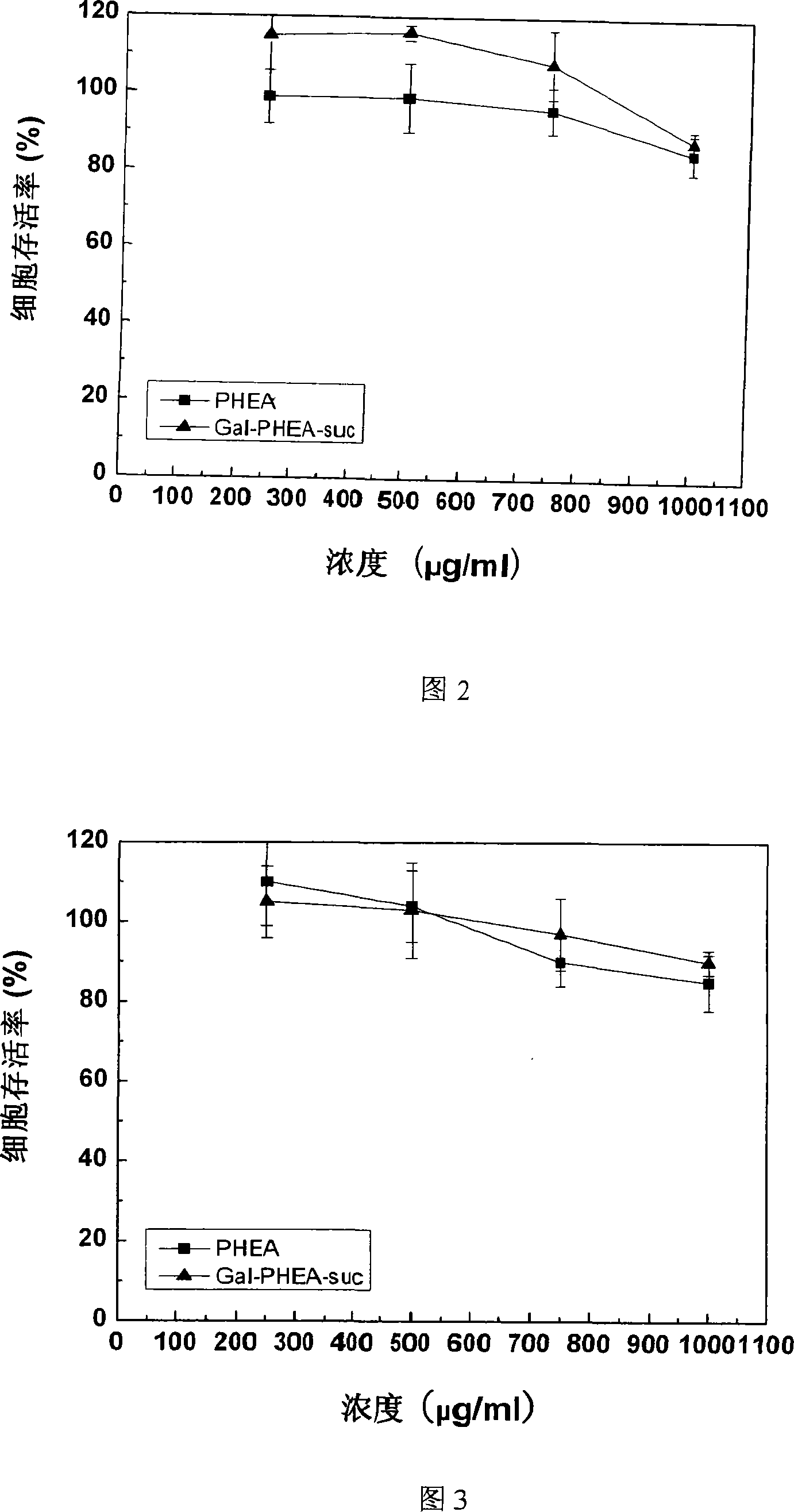
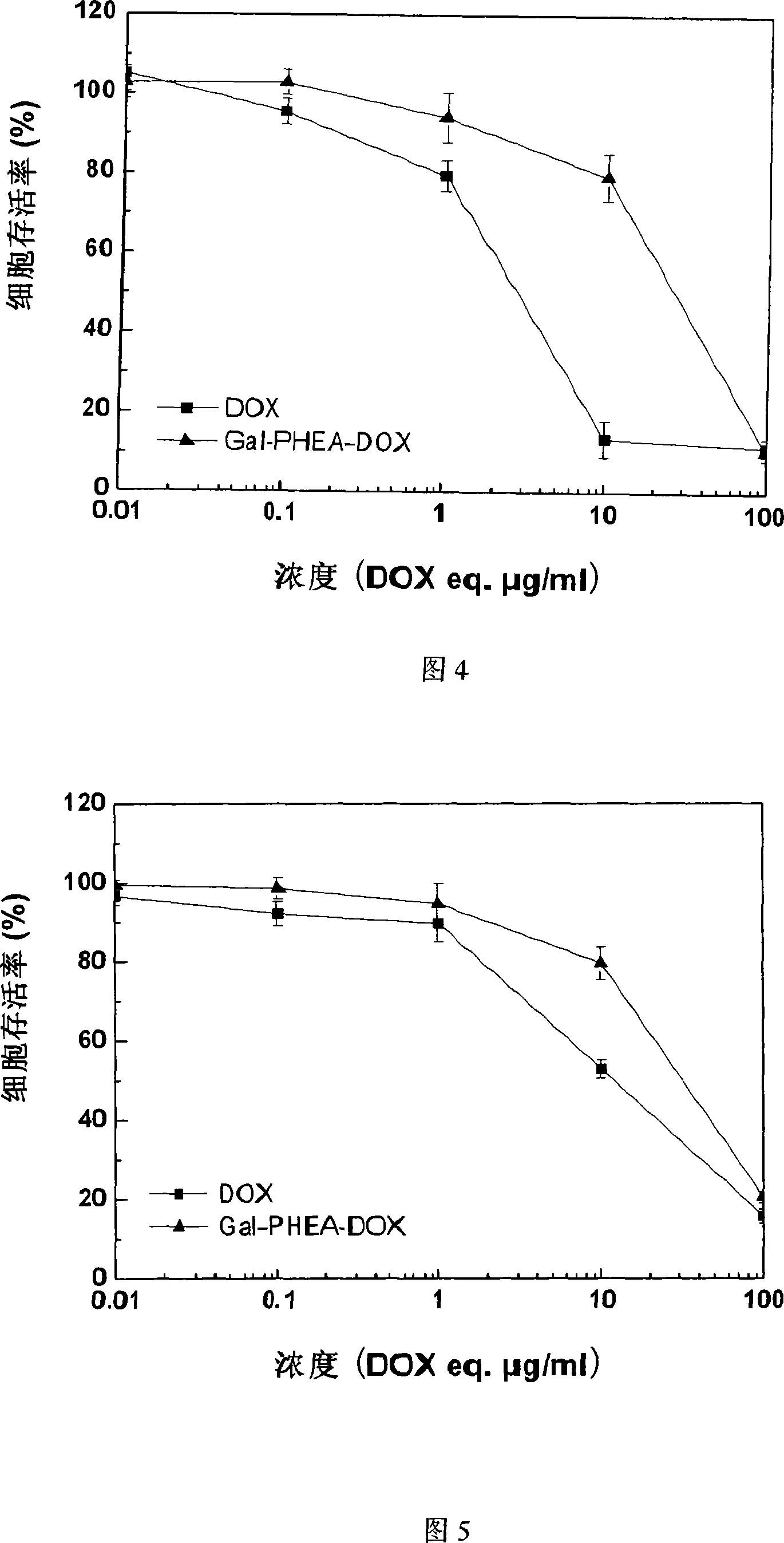
Method for preparing coupled article of polyasparamide derivant and adriablastina, and uses thereof
ActiveCN101195032AEasy to retouchRaw materials are easy to getOrganic active ingredientsPharmaceutical non-active ingredientsSide effectAcyl group
The invention belongs to the biomedical technical field, and in particular relates to a synthesis of targeting macromolecular drug carriers and a synthesis of macromolecular pro-drug after the pro-drug is coupled with anti-cancer drug adriamycin. The carrier relates to a derivative of alpha and beta-polymer-(2-hydroxyethyl)-L-asparagine, modifies targeting group galactosyl and active group succinyl acyl on hydroxy group, and is a safe drug carrier with hepar targeting. The macromolecular pro-drug which is coupled with anti-cancer drug adriamycin reserves the antineoplastic function of adriamycin, lowers the toxic and side effect of adriamycin to bodies, and can be developed into one adriamycin pro-drug with hepar targeting.
Owner:合肥硕健医药科技有限公司
Negative-pressure transdermal drug delivery device
The invention provides a negative-pressure transdermal drug delivery device and aims to solve the problems that a conventional transdermal drug delivery device injures skin in the use process and is low in transdermal drug delivery curative effect. The technical key points are that a composite drug-loaded dressing containing a drug is loaded into the negative-pressure transdermal drug delivery device, a meshy elastic member fixed at the edge of the inner wall is arranged in the position, in contact with the skin, of the negative-pressure transdermal drug delivery device, and a semi-circular member allowing air to pass is arranged in the internal middle of the negative-pressure transdermal drug delivery device. After negative pressure is pumped into the negative-pressure transdermal drug delivery device, the surface area of the skin per unit area is increased, the skin tissue space is enlarged, and further, the drug transdermal impedance is reduced. Along with increase of the negative pressure, the meshy elastic member and the semi-circular member in the negative-pressure transdermal drug delivery device limits swelling of the skin and tissues, a reversed pushing action force is formed on the skin in negative pressure due to action of resistance, so that the drug can be easily loaded into the skin and enters a human body through the space-enlarged skin and tissues, and after relief of the negative pressure, the human body skin tissues recover while the drug remains in the human body.
Owner:柴雪青
Liposomes with improved drug retention for treatment of cancer
The present invention relates to the use of copper ions to achieve enhanced retention of a therapeutic agent within a liposome. The invention may be employed to more effectively deliver a liposomally encapsulated therapeutic agent to a target site in vitro and in vivo for anti-cancer or other therapy. The liposome may comprise an interior buffer solution containing the therapeutic agent, the solution having a pH less than 6.5 and most preferably approximating pH 3.5. At least some of the copper ions are retained within the interior solution. In a particular embodiment the therapeutic agent may be a chemotherapeutic drug, such as irinotecan. The invention may also comprise an ionophore to facilitate loading of drug into the liposome. In one particular embodiment the combination of the ionophore A23187 and encapsulated divalent copper (Cu2+) resulted in an irinotecan formulation that exhibited surprisingly improved drug retention attributes.
Owner:BRITISH COLUMBIA CANCER AGENCY BRANCH
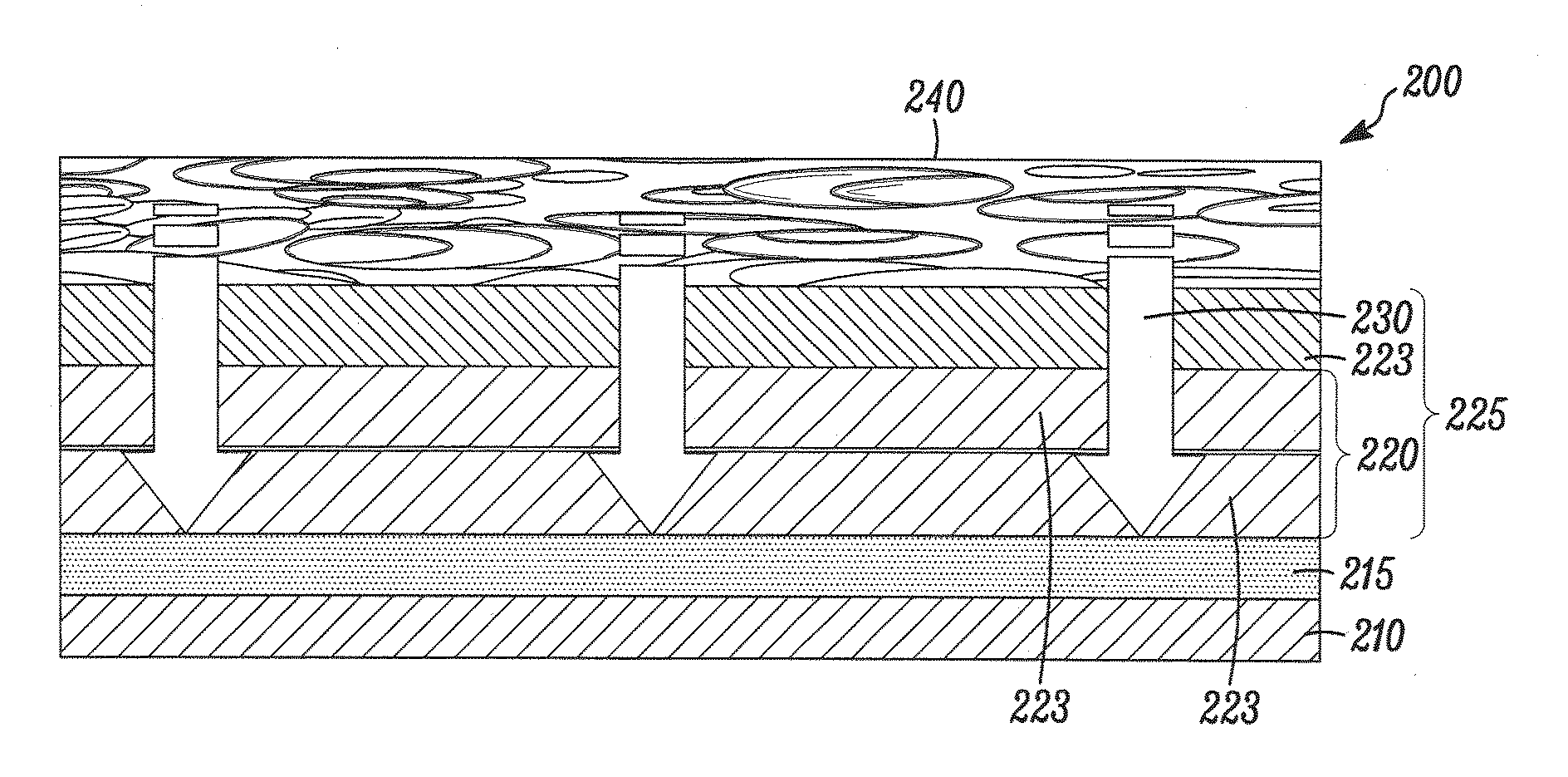
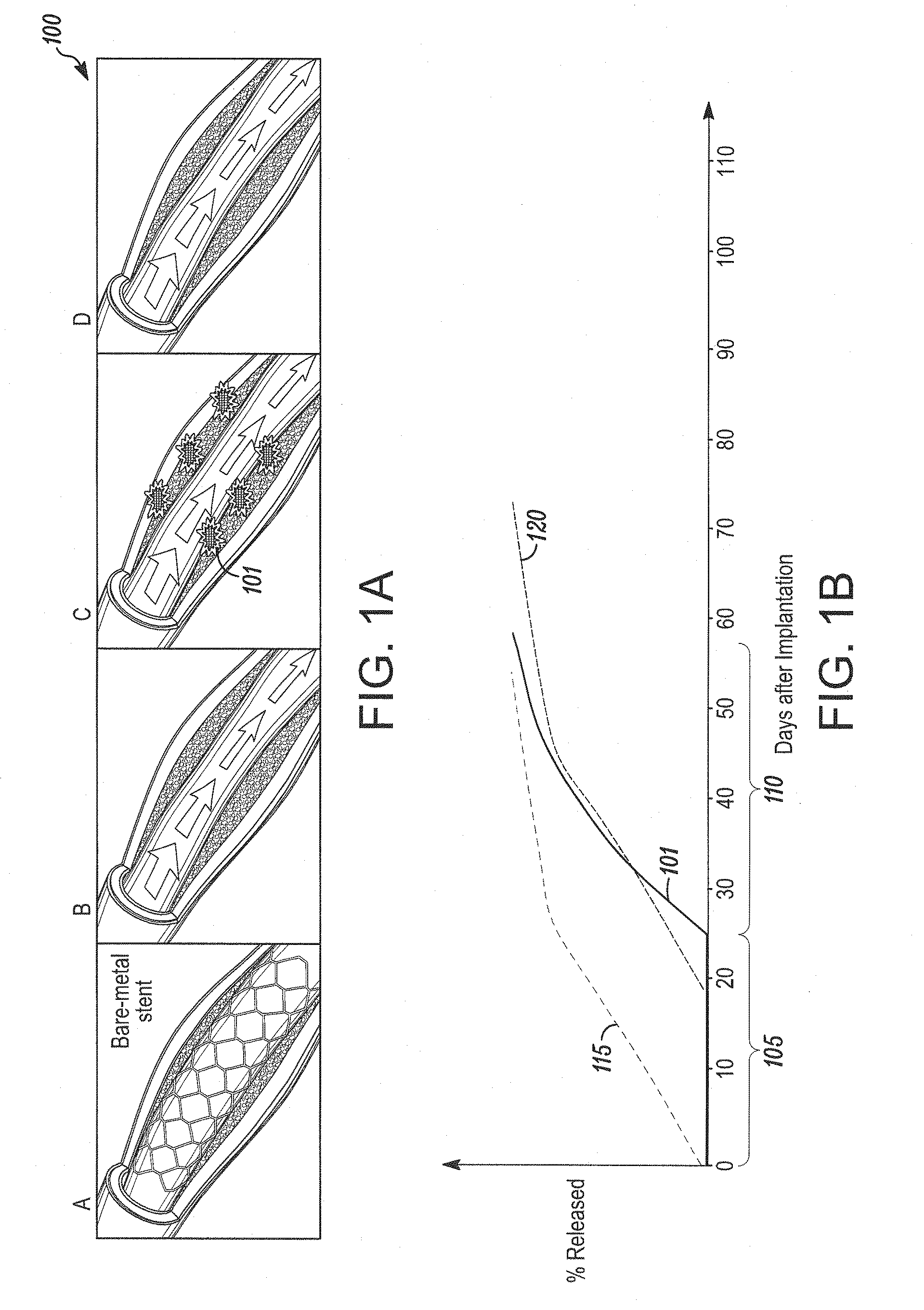
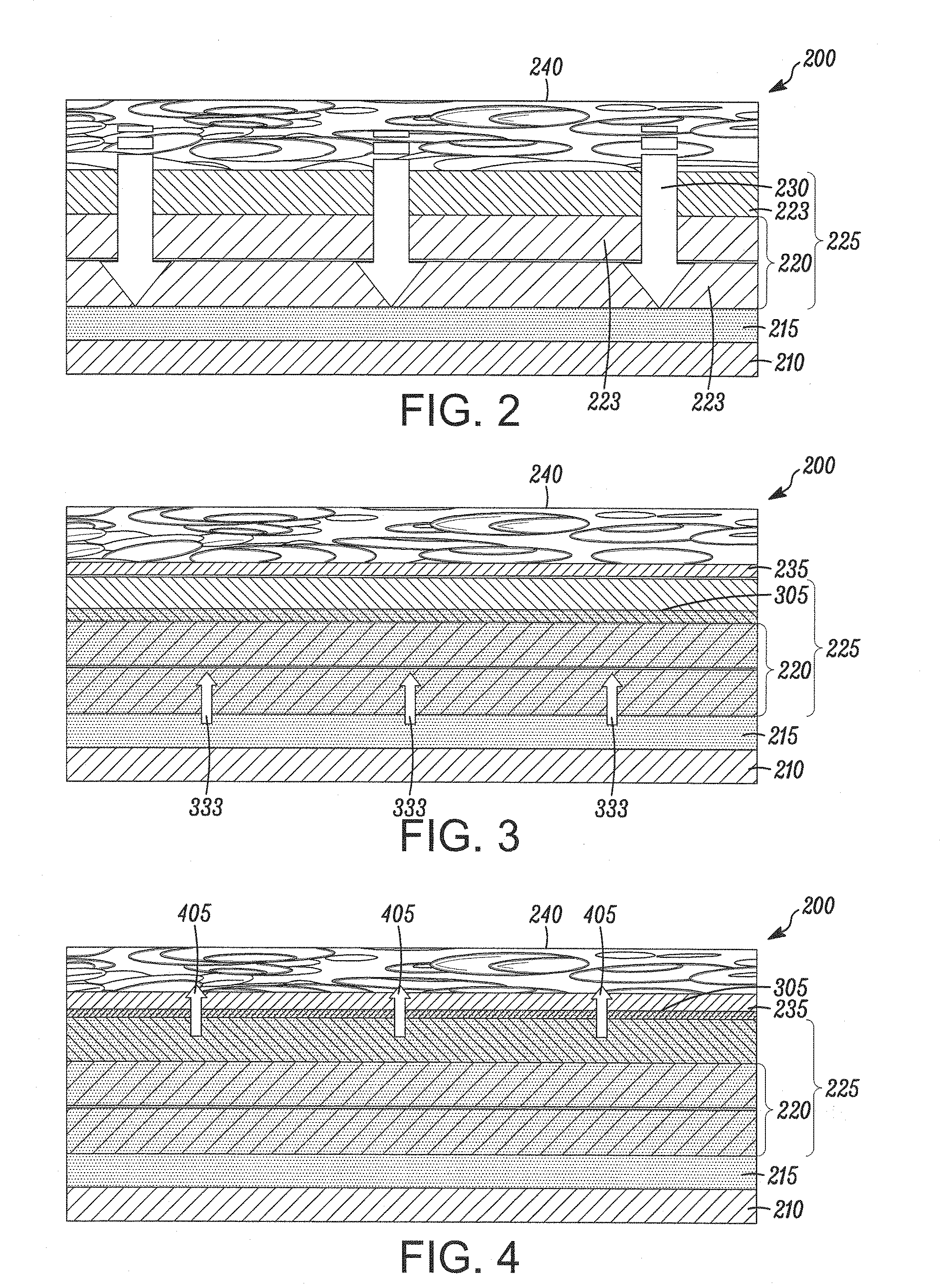
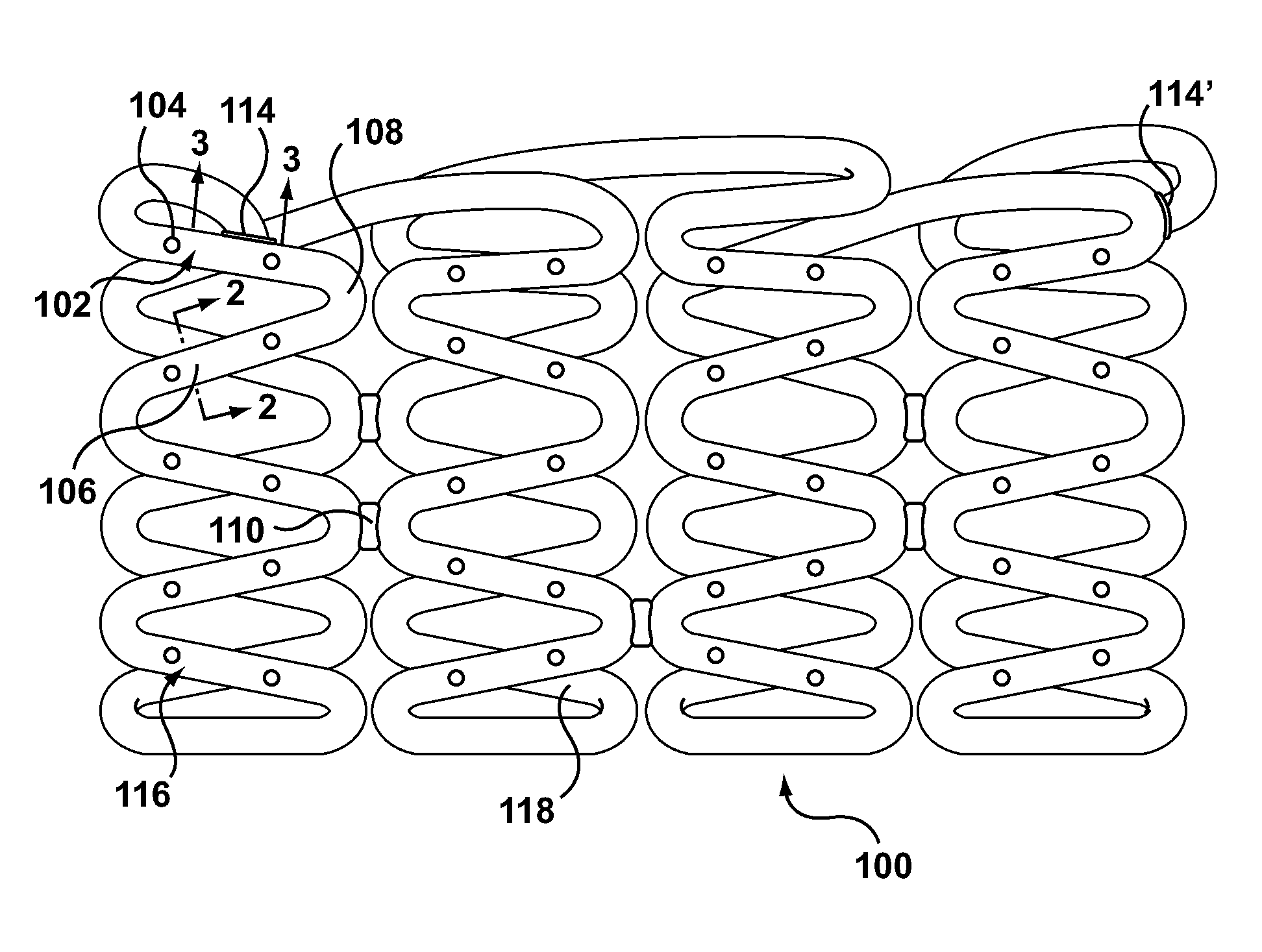
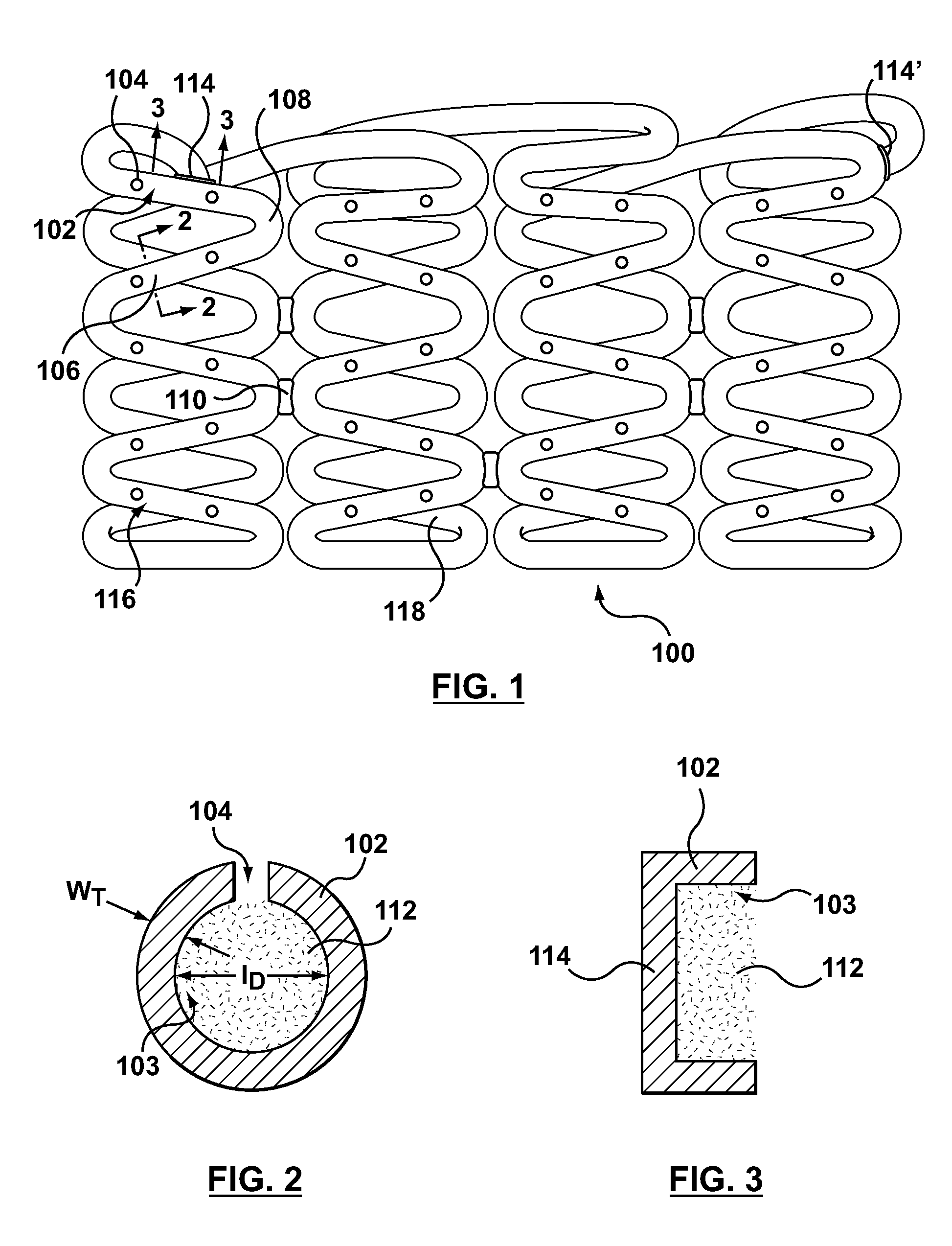
Bare metal stent with drug eluting reservoirs having improved drug retention
ActiveUS20120109284A1Reduced elutionMaintain vessel patencyStentsMetal rolling stand detailsBare-metal stentInsertion stent
Implantable medical devices may be utilized to locally delivery one or more drugs or therapeutic agents to treat a wide variety of conditions, including the treatment of the biological organism's reaction to the introduction of the implantable medical device. These therapeutic agents may be released under controlled and directional conditions from a stent having reservoirs so that the one or more therapeutic agents reach the correct target area, for example, the surrounding tissue. Features may be incorporated into the walls and bases of these reservoirs to improve securement of the drug construct.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Pharmaceutical gastro-retentive solid oral dosage form of nilotinib
The present invention relates to a pharmaceutical gastro-retentive solid oral dosage form comprising nilotinib as the active ingredient. The invention is further related to methods of preparing said dosage form.
Owner:RANBAXY LAB LTD
Liposomal formulations of anthracycline agents and cytidine analogs
Compositions which comprise an anthracycline agent, and a cytidine analog are encapsulated in liposomal carriers. The preferred anthracycline agent is selected from the group of daunorubicin, doxorubicin, and idarubicin, while the preferred cytidine analog is selected from the group of cytarabine, gemcitabine, or 5-azacytidine. The combination of the anthracycline agent and cytidine analog encapsulated in said liposomal carriers are useful in achieving a drug retention and a sustained drug release for each therapeutic agent.
Owner:CELATOR PHARMA INC
Composition containing toad venom extraction used for pain relieving and anesthesia
InactiveCN1830457AStop lossProlong the action timeAmphibian material medical ingredientsNervous disorderToad VenomMolecular materials
A composition containing the extract of toad venom for anodyne and anesthesia is disclosed, which features that it contains also high-molecular materials, so having slow release function.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Liposome compositions for improved drugretention
Owner:ALZA CORP
Pharmaceutical preparation for rectal administration
InactiveUS20060147541A1Eliminate side effectsReduce dosing frequencyPowder deliveryOrganic active ingredientsMicroparticleWater soluble
A pharmaceutical preparation for rectal administration by which a drug is retained in the affected region or the lower region of the rectum, where the drug is sustained-released is disclosed. The present invention is a pharmaceutical preparation for rectal administration comprising a coated drug-supported particle dispersed in a base, wherein the coated drug-supported particle is what a drug-supported porous microparticulate carrier is coated with a water-soluble polymer having a certain viscosity.
Owner:SATO PHARMA
Composition containing toad venom extraction used for pain relieving and anesthesia
InactiveCN1830457BStop lossProlong the action timeAmphibian material medical ingredientsNervous disorderToad VenomMolecular materials
A composition containing the extract of toad venom for anodyne and anesthesia is disclosed, which features that it contains also high-molecular materials, so having slow release function.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
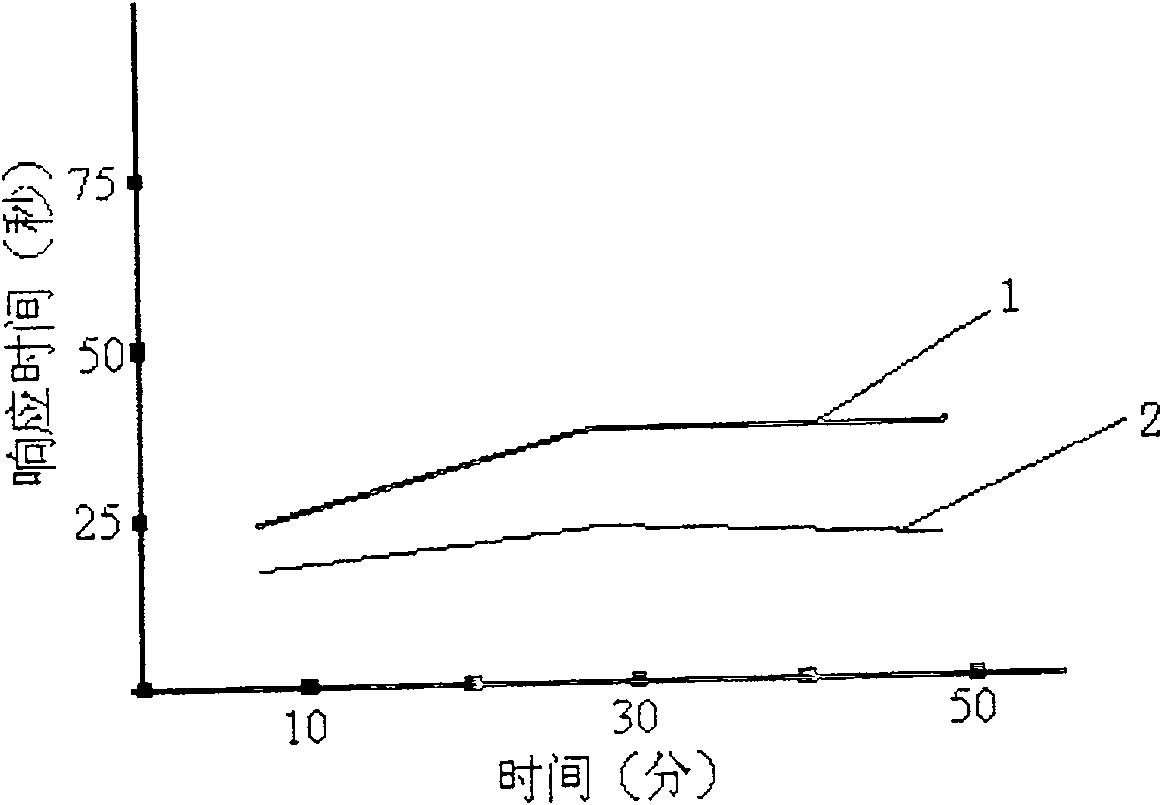
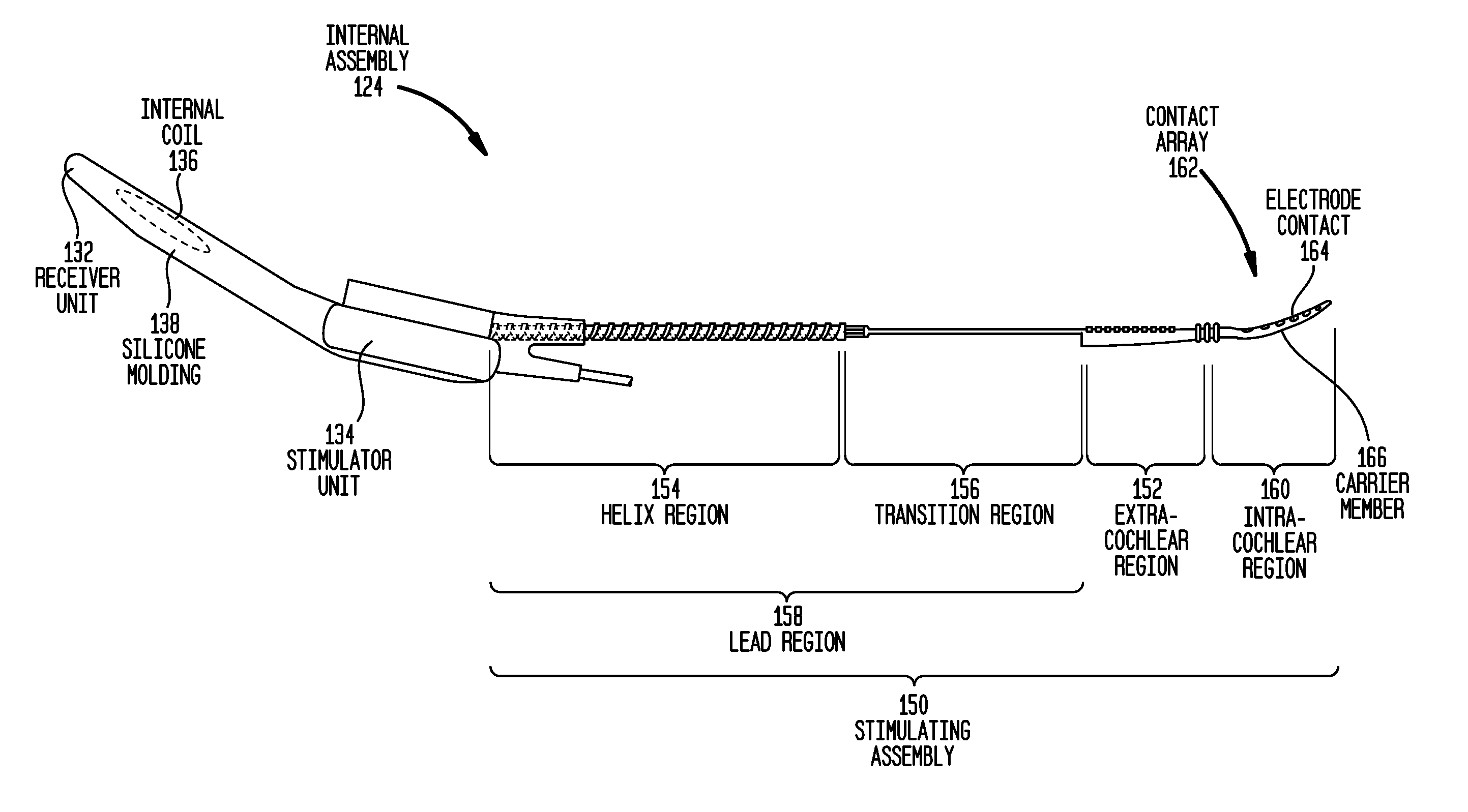
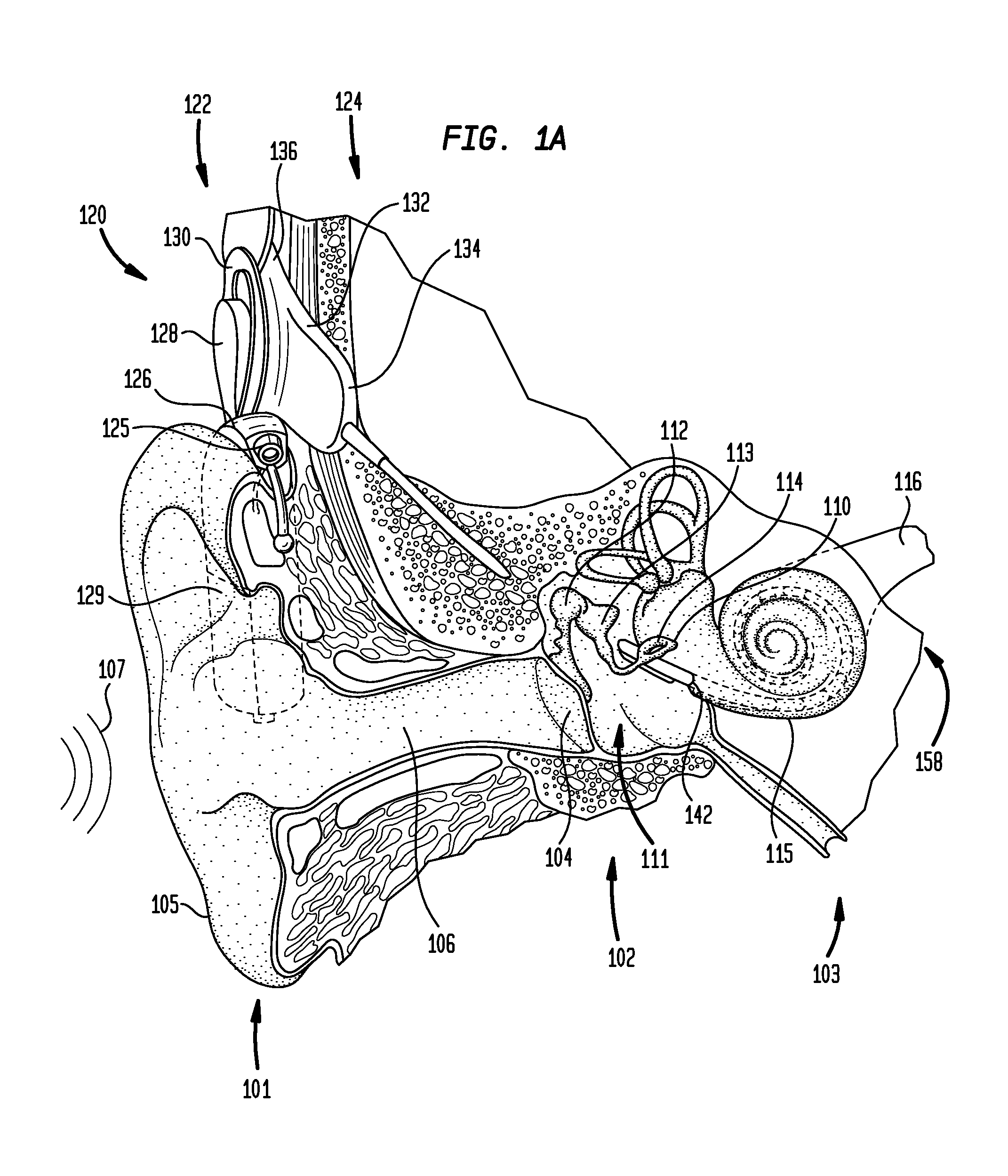
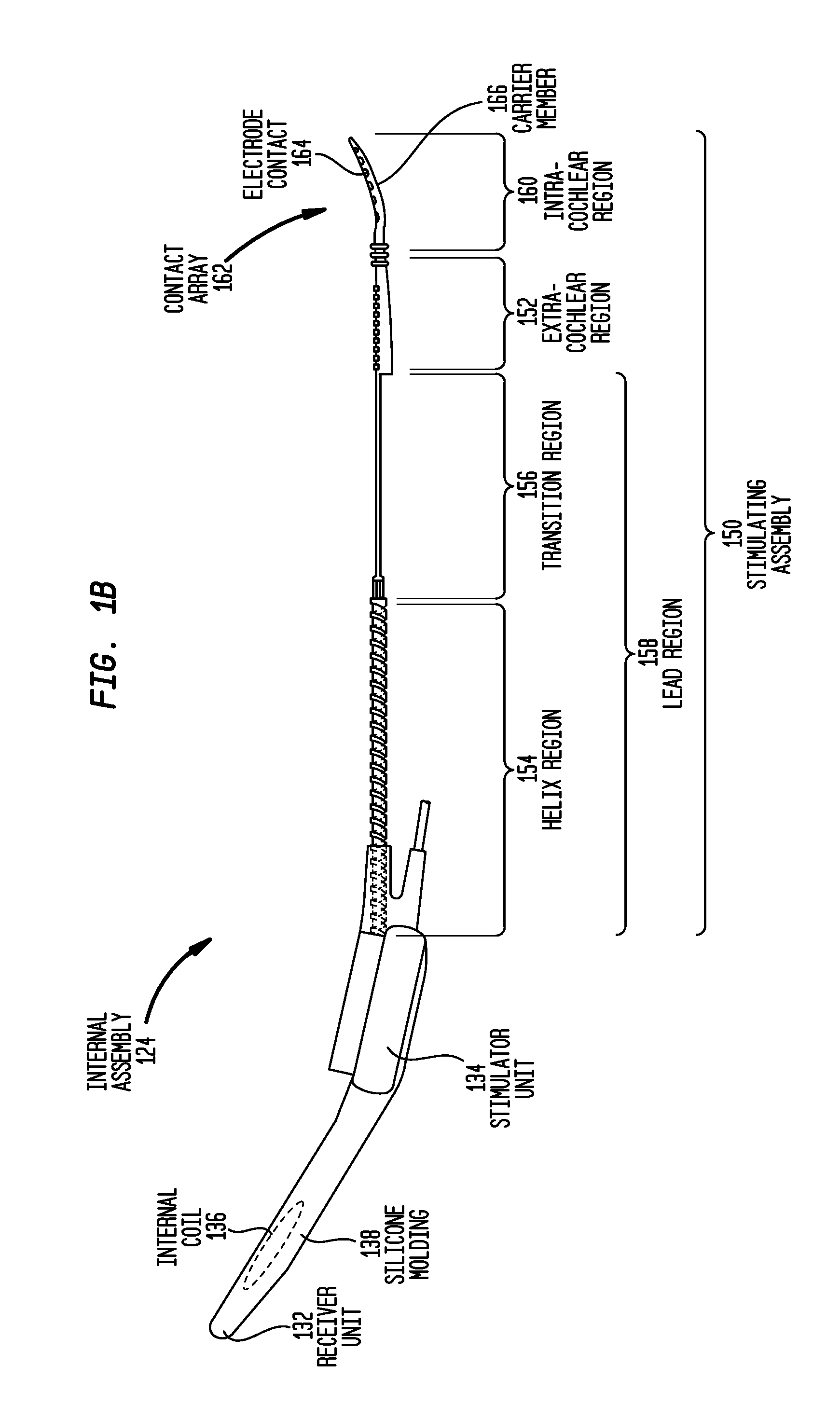
Drug retaining surface features in an implantable medical device
An implantable component of a medical device, comprising a polymeric surface. The component includes one or more macro-surface features at the polymeric surface having a configuration that, following application of a liquid drug to the surface retains a quantity of the liquid drug adjacent the surface.
Owner:COCHLEAR LIMITED
Medical device loaded with formulation for targeted delivery of biologically active material/s and method of manufacture thereof
The present invention relates to targeted drug delivery of a drug or therapeutic agent through medical devices coated with formulations comprising of therapeutic agent. The coating on medical devices comprises of therapeutic agent(s), affinity vehicle(s) and additives for targeted drug delivery of biologically active material(s). The invention provides a method of manufacturing the formulation, method of coating the medical devices with such formulations to achieve controlled delivery of optimum drug dose at the target site within the body, desirable drug retention on the medical devices in vivo and in vitro and desirable drug release at the target tissue in-vivo. The invention this provides a mechanism to enhance the bioavailability of the therapeutic agent at the target tissue in the treatment of restenosis thereby reduces the actual dose of the therapeutic agent and provides a very thin layer of coating on the surface of the medical device.
Owner:MERIL LIFE SCI PVT
Amphiphilic block copolymers for delivery of active agents
ActiveUS11007148B2Cyclic peptide ingredientsPharmaceutical non-active ingredientsMethacrylateVinyl ether
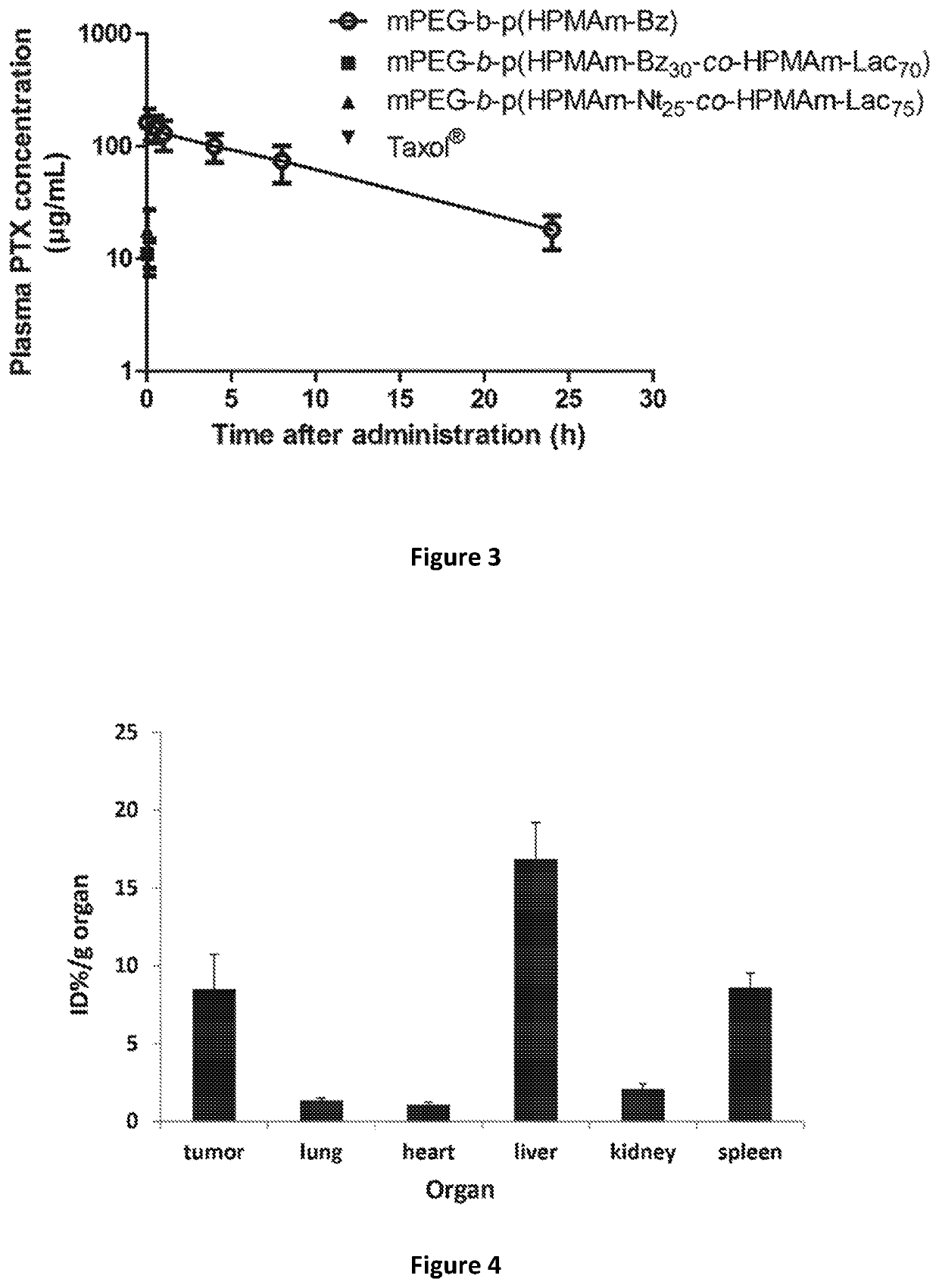
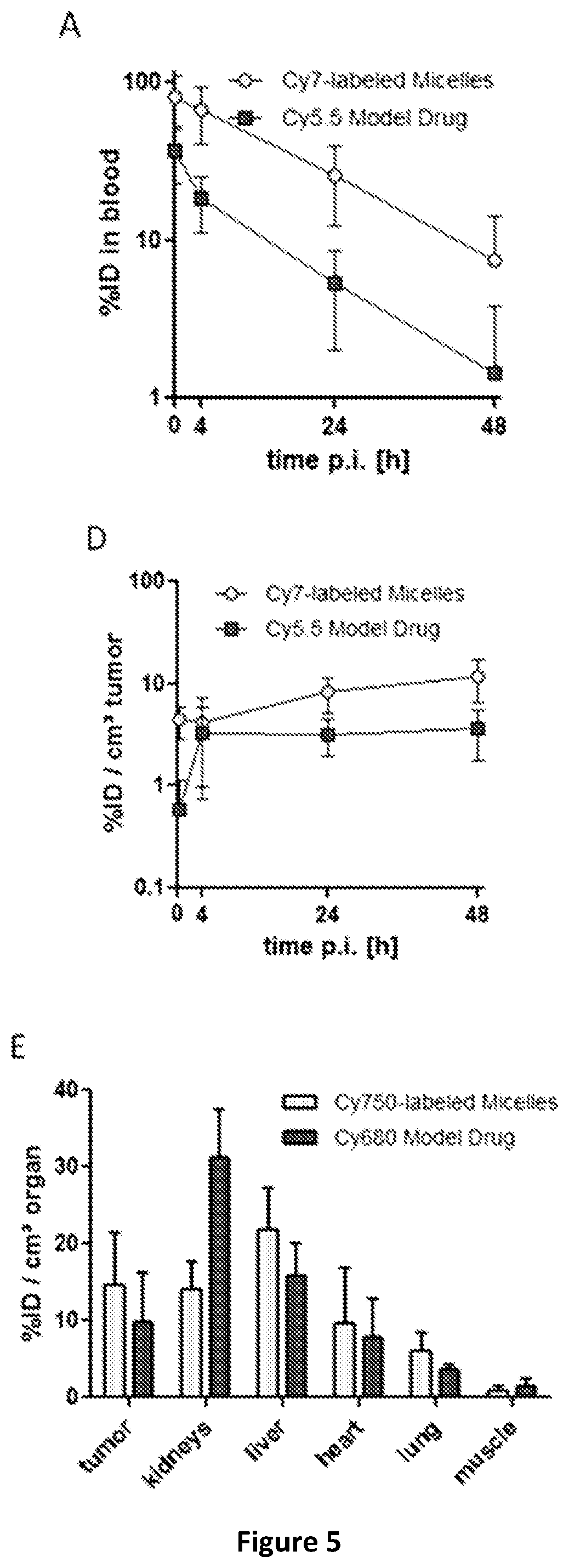
The present invention provides an amphiphilic block copolymer for drug delivery and a controlled release system comprising the same copolymer. The copolymer comprises at least one hydrophilic block and at least one hydrophobic block, wherein the hydrophobic block is selected from the group consisting of acrylates, methacrylates, acrylamides, methacrylamides, vinylethers and their aromatic derivatives, wherein at least one hydrophobic block contains an aryl group, which is bound to the hydrophobic block with a degradable linker. The controlled release system of the invention shows high stability and high drug retention when administered in vivo enabling accumulation and drug release at the site of action.
Owner:HALLIBURTON ENERGY SERVICES INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com