Method for preparing coupled article of polyasparamide derivant and adriablastina, and uses thereof
A technology of polyasparagine and derivatives is applied in the field of biomedicine to achieve the effects of retaining anticancer activity, good water solubility, and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of polyasparagine derivative (Gal-PHEA-suc)
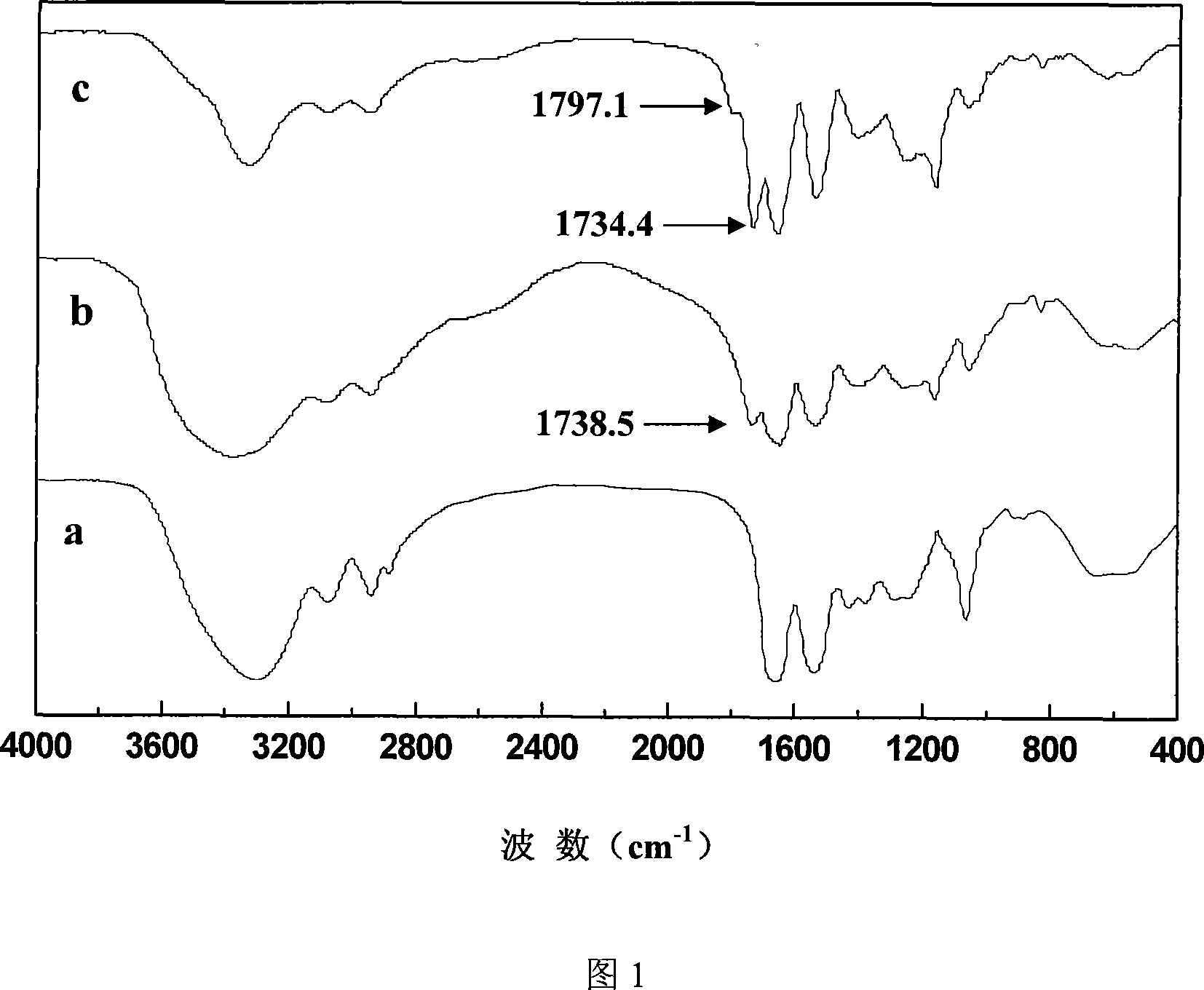
[0032] Weigh 2.5 g of PHEA and dissolve it in 100 ml of DMF, add 2.5 g of succinic anhydride, react at room temperature for 6 hours, then dialyze with distilled water and freeze-dry to obtain PHEA-Suc. Infrared spectrum analysis is shown in Figure 1, the product has a characteristic peak ν c=o : 1738.5cm -1 , ν as c-o-c : 1164.7cm -1 , Elemental analysis shows that the substitution degree of succinic acid group is 43%.
[0033] Weigh 2 grams of N,N'-carbonyldiimidazole (CDI) and 1.5 grams of lactobionic acid and dissolve them in 30ml of anhydrous DMF, add the CDI solution to the lactobionic acid solution drop by drop at 0°C, and keep it for 4 hours; weigh 1 g of PHEA-suc, dissolved in 20 ml of anhydrous DMF, was added dropwise to the above solution, reacted at 0°C for 15 minutes, added a few drops of triethylamine as a catalyst, and stirred at room temperature for four days. Then it was dia...
Embodiment 2
[0034] Example 2: Preparation of polyasparagine derivative-doxorubicin conjugate (Gal-PHEA-DOX)
[0035]Weigh 0.5g EDC and dissolve in 20ml DMF, 0.125g DOX, stir to dissolve. 0.5 g Gal-PHEA-suc was dissolved in 10 ml DMF, then mixed with the above-mentioned doxorubicin solution, and stirred at room temperature for 24 hours in the dark. The reaction product was dialyzed with double distilled water for 4 days, and freeze-dried to obtain Gal-PHEA-DOX. This process was also carried out in the dark. The doxorubicin content of Gal-PHEA-DOX detected by ultraviolet and visible light was 9.7wt%.
Embodiment 3
[0036] Example 3: Inhibition of Gal-PHEA-suc and Gal-PHEA-DOX on the growth of human cervical cancer cell Hela and liver cancer cell HepG2
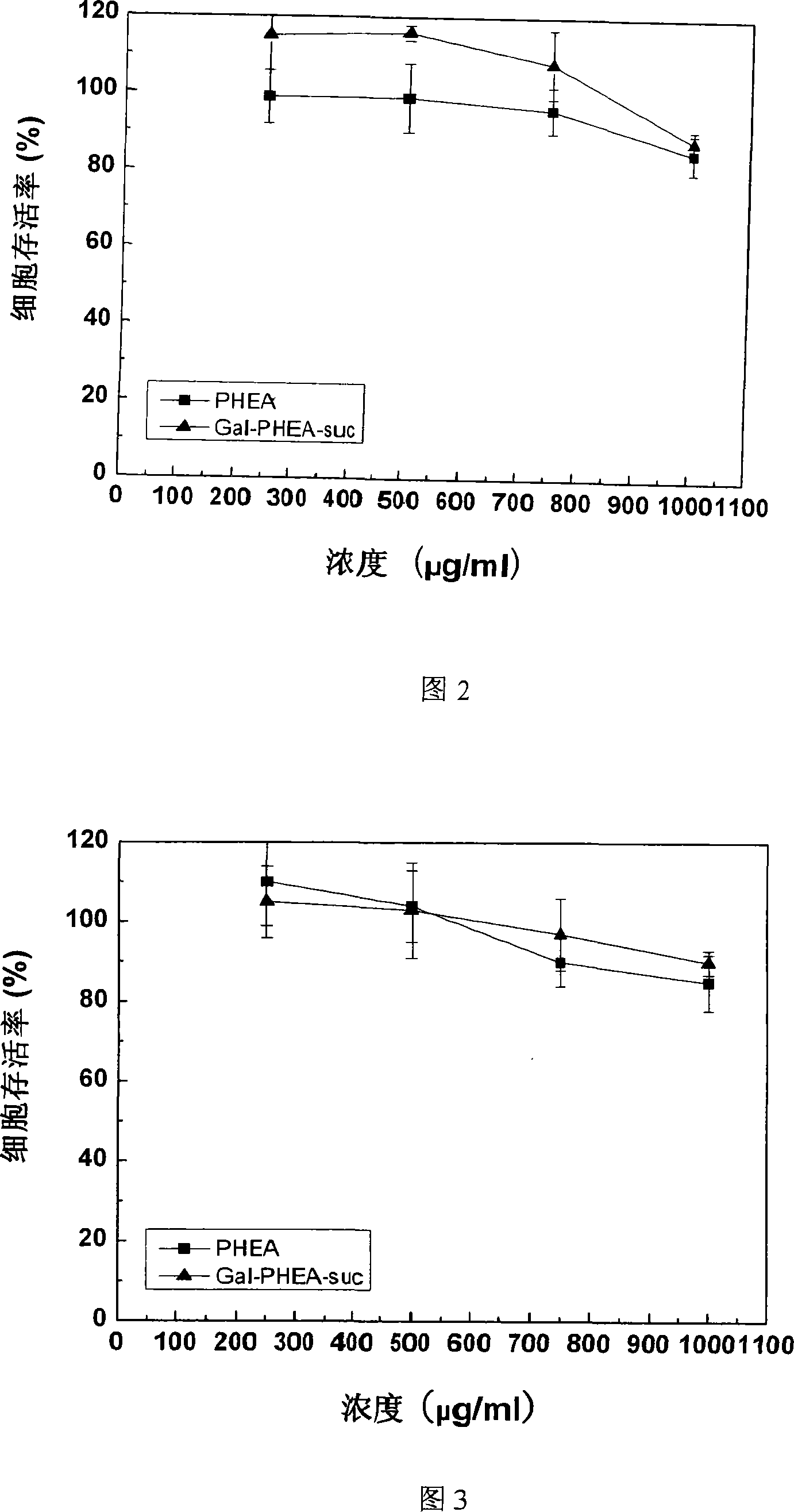
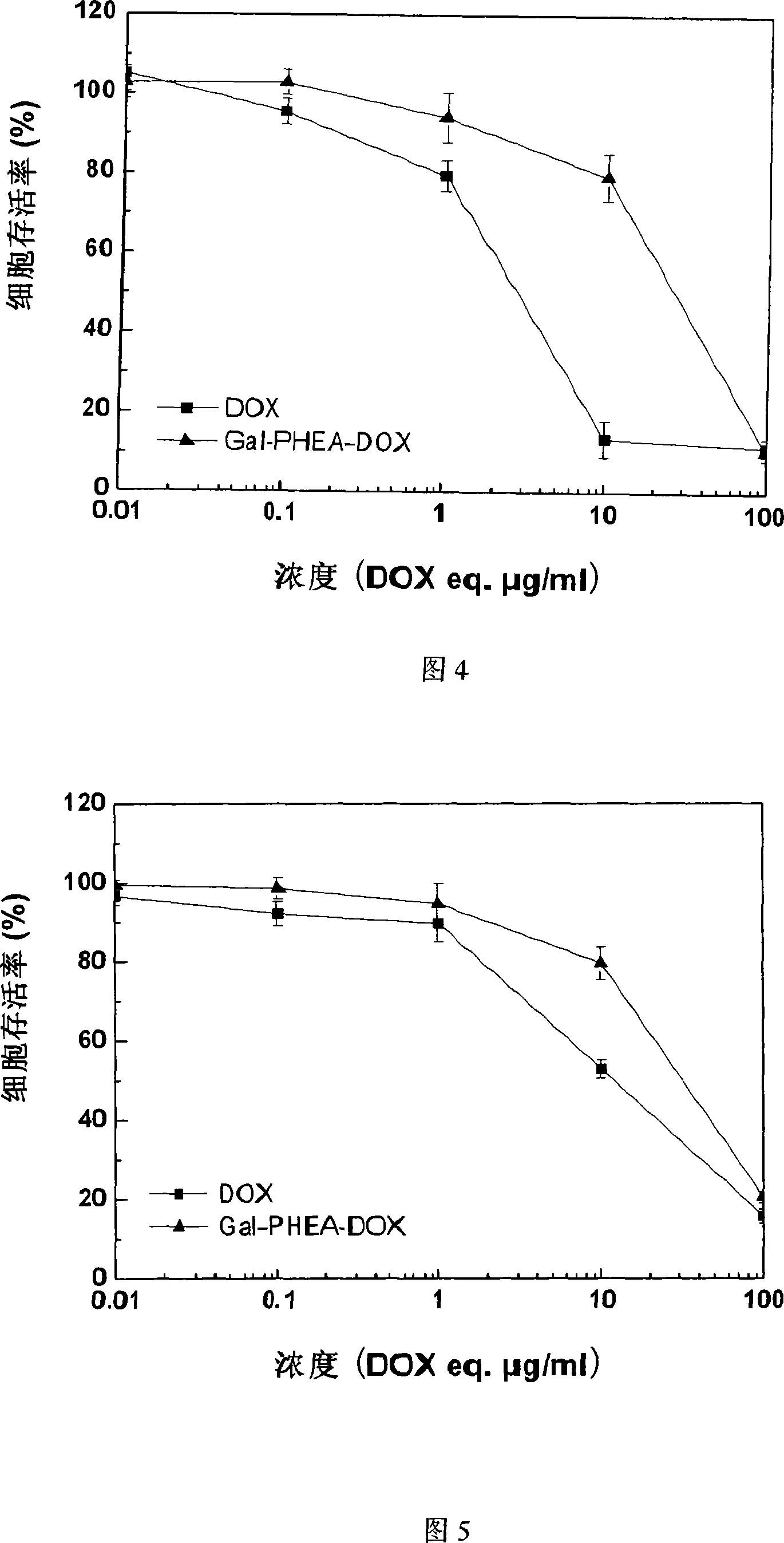
[0037] Press 1×10 4 Density per well Transfer the Hela and HepG2 cells in the logarithmic growth phase to 96-well plates, add 100 μl 1640 medium (10% serum + double antibody), and culture for 24 hours; take PHEA, Gal-PHEA-suc , DOX and Gal-PHEA-DOX were diluted with culture medium to a preset concentration gradient, and 100 μl was added to each well, and cultured in a 37°C incubator; the culture was terminated after the set time, and the cell viability was measured by the MTT method. Figure 2 and Figure 3 show that after 24 hours of action, PHEA and Gal-PHEA-suc have no toxicity to Hela and HepG2 when the concentration is lower than 0.75mg / ml, and the cytotoxicity is still very small when the concentration rises to 1mg / ml. Figure 4 and Figure 5 show that after being incubated with cells for 48 hours, Gal-PHEA-DOX has an inhibitory effect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com