Methods for administration of antibiotics
A method of administration and technology of antibiotics, which can be used in antibacterial drugs, anti-infective drugs, pharmaceutical formulations, etc., and can solve problems such as low toxicity and unpredictability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Study A:C max Effects on CPK and skeletal muscle toxicity
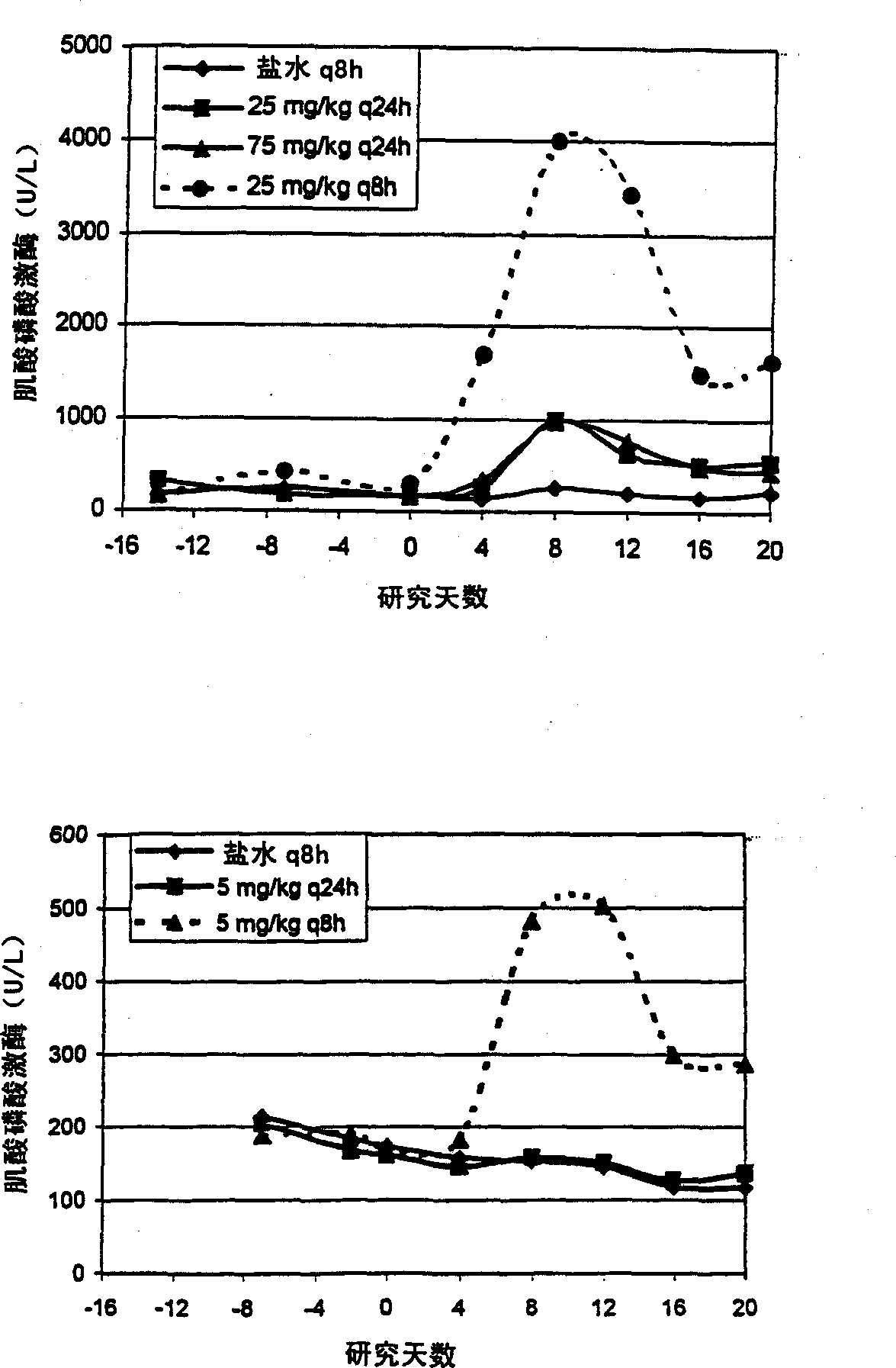
[0037] In order to study C max Effects on Skeletal Muscle Toxicity, Dogs (4 male dogs / group) administered intravenously with saline q8h, daptomycin 25mg / kg q24h, daptomycin 75mg / kg q24h and daptomycin 25mg / kg q8h20 sky. Skeletal muscle toxicity was determined by increases in dog CPK levels above the normal range and by microscopic changes in bone tissue.
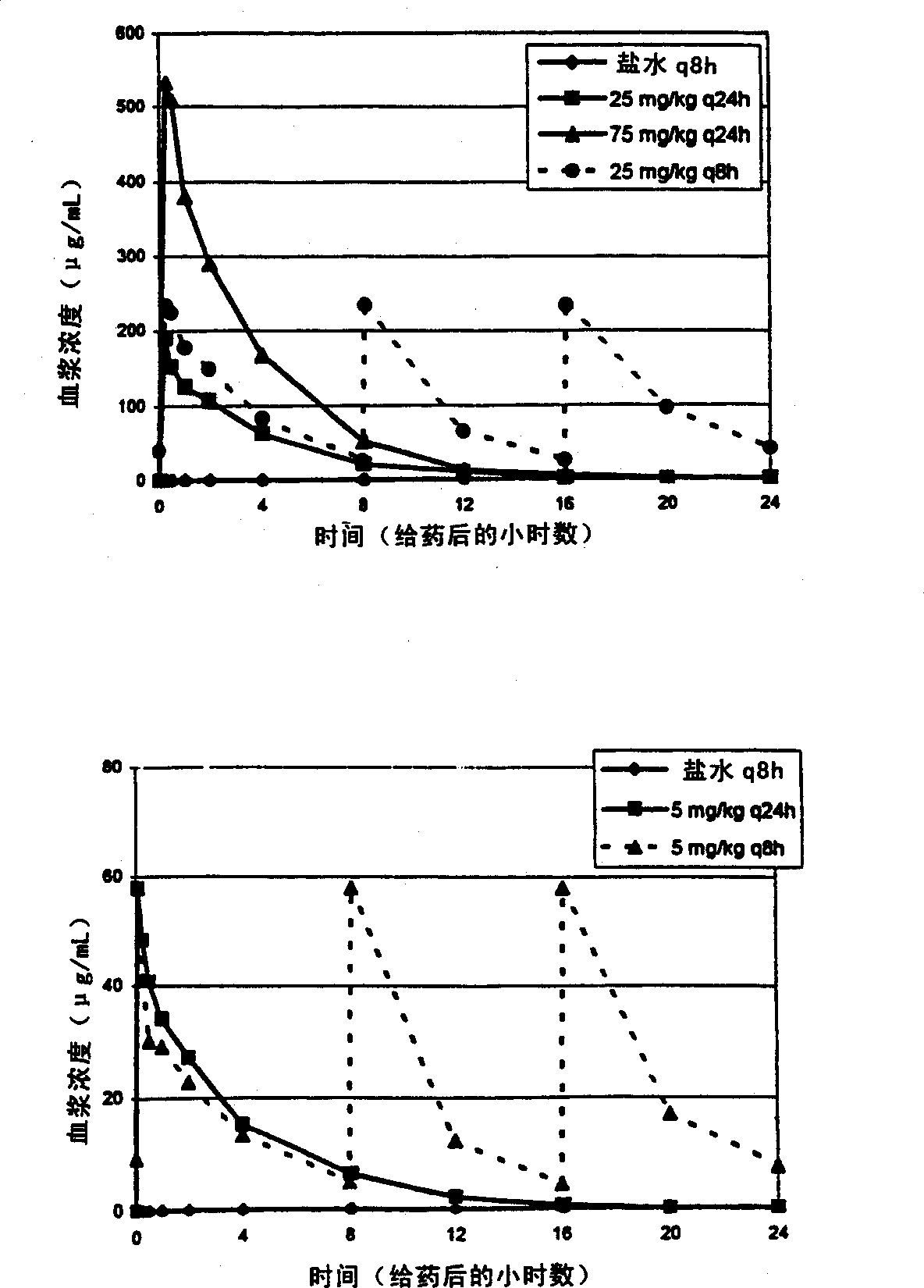
[0038] Steady-state plasma concentrations of daptomycin at day 18 of dosing were determined by HPLC. C at 25 mg / kg q8h compared to 25 mg / kg q24h max The levels are essentially the same (1.23 times higher). C at 75 mg / kg q24h max Levels were 2.8-fold at 25 mg / kg q8h. See attached figure 1 The upper panel of (Study A). AUC at 25 mg / kg q8h was essentially the same (0.37-fold higher) compared to 75 mg / kg q24h (see Table 2 and appendix figure 2 above picture).
[0039] There was a dose-proportional increase in peak CPK activity when the dose ...
Embodiment 2
[0049] Example 2 Study B: Effect of Plasma Concentration Thresholds on Skeletal Muscle Toxicity
[0050] To study the effect of threshold plasma concentrations on skeletal muscle toxicity, dogs (4 male dogs / group) were administered intravenous saline q8h, daptomycin 5 mg / kg q24h (approximate NOEL q24h) and daptomycin 5 mg / kg q8h Program 20 days.
[0051] Steady-state plasma concentrations of daptomycin at day 18 of dosing were determined by HPLC as described in Example 1. The q8h interval represents the 3 half-lives in the dog (t 1 / 2 = 2.5 hours) and respond to steady state C max has the lowest impact. 5mg / kg q24h and 5mg / kg q8h of C max It is the same for both dosing regimens. See attached figure 1 The lower panel of (Study B). However, the AUC at 5 mg / kg q8h was about 3 times (2.6 times) that at 5 mg / kg q24h (see Table 4 and appendix figure 2 the figure below).
[0052]Serum CPK levels were determined as disclosed in Example 1. CPK levels at 5 mg / kg q24h were unch...
Embodiment 3
[0061] To study the C of quinupristin / dalfopristin max For skeletal muscle toxicity, dogs (4 male dogs / group) were given intravenous saline q8h, quinupristin / dalfopristin 25mg / kgq24h, quinupristin / dalfopristin 75mg / kg q24h, and quinupristin The dosing regimen of nupristin / dalfopristin 25mg / kg q8h for 20 days.
[0062] Steady-state plasma concentrations of quinupristin / dalfopristin at day 18 of dosing were determined by HPLC. The C of 25 mg / kg q8h, 25 mg / kg q24h and 75 mg / kg q24h was determined as described in Example 1 max levels and AUC. Similarly, CPK levels and the incidence of muscle-related histopathological findings were determined as described in Example 1 at 25 mg / kg q8h, 25 mg / kg q24h and 75 mg / kg q24h. For skeletal muscle, 6 sites were examined for each of 4 dogs, for a total of 24 sites. If no microscopic myopathy or effects on CPK levels are observed at any dose regimen, the dose may be increased. For example, C at 50 mg / kg q8h, 50 mg / kg q24h and 150 mg / kg q24...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com