Hemostatic system for body cavities
a technology of hemostatic system and cavity, which is applied in the field of medical devices, can solve the problems of difficult control of epistaxis related to a patient's nasal passageway, easy uncontrolled bleeding of nasal passageways, and easy bleeds of nasal passageways, so as to facilitate hemostasis and facilitate the later removal. , the effect of facilitating or enhancing blood clot formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
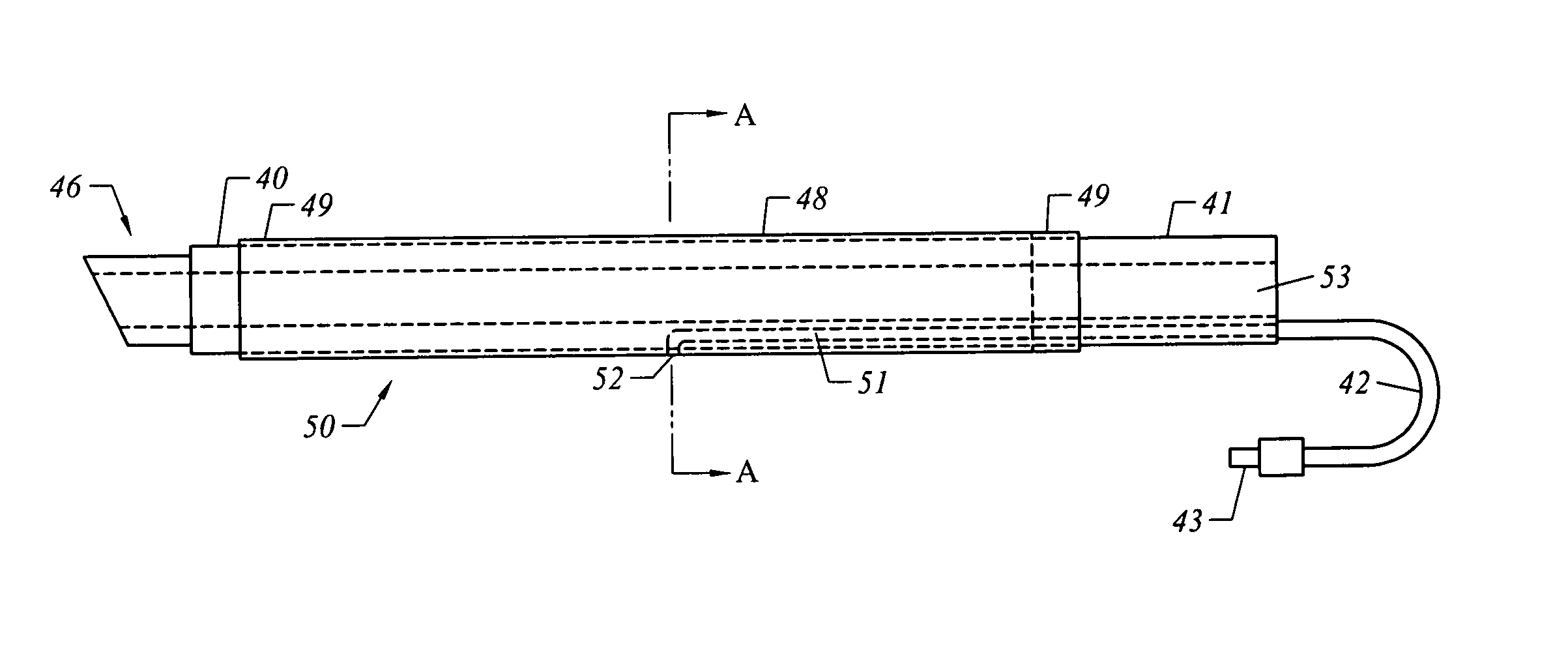
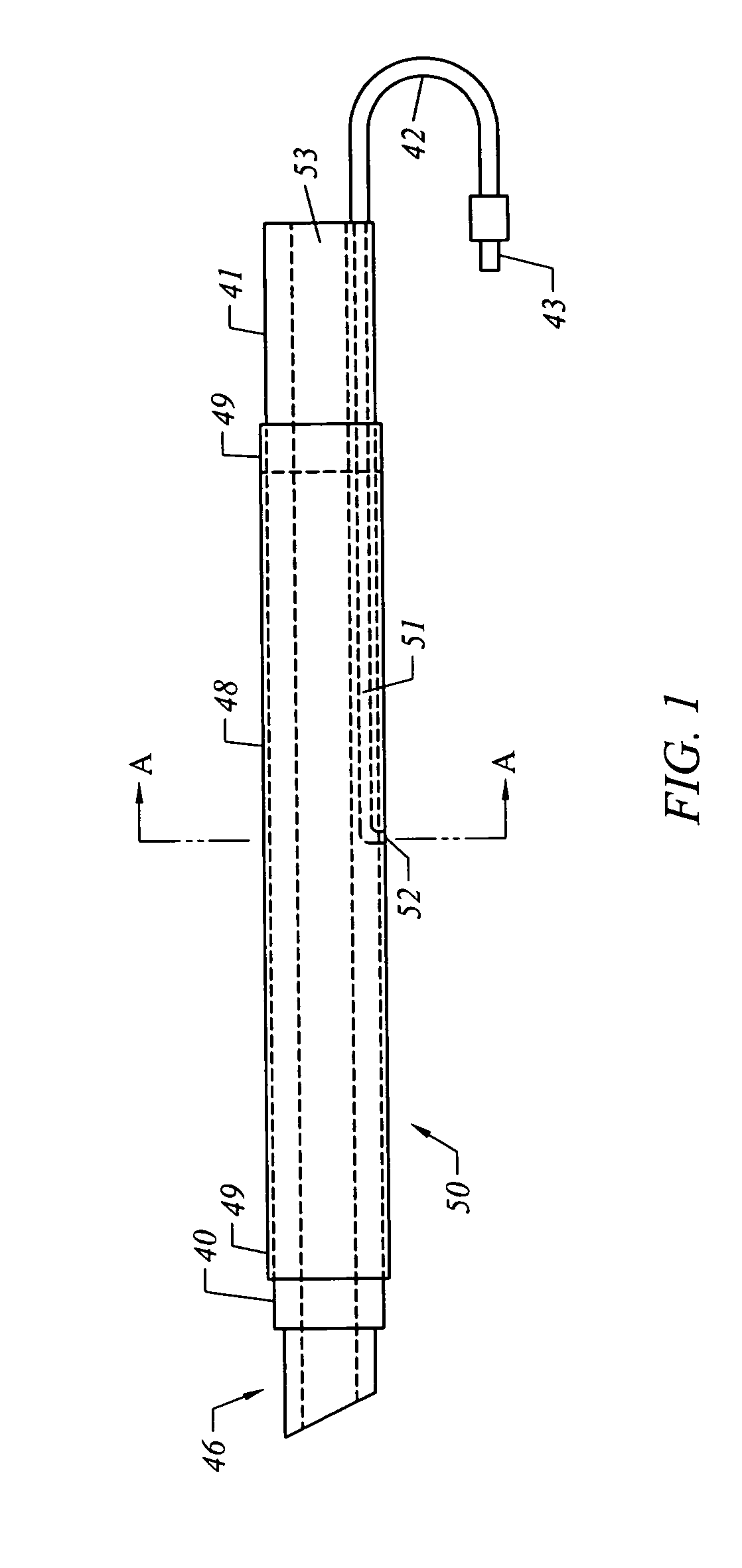
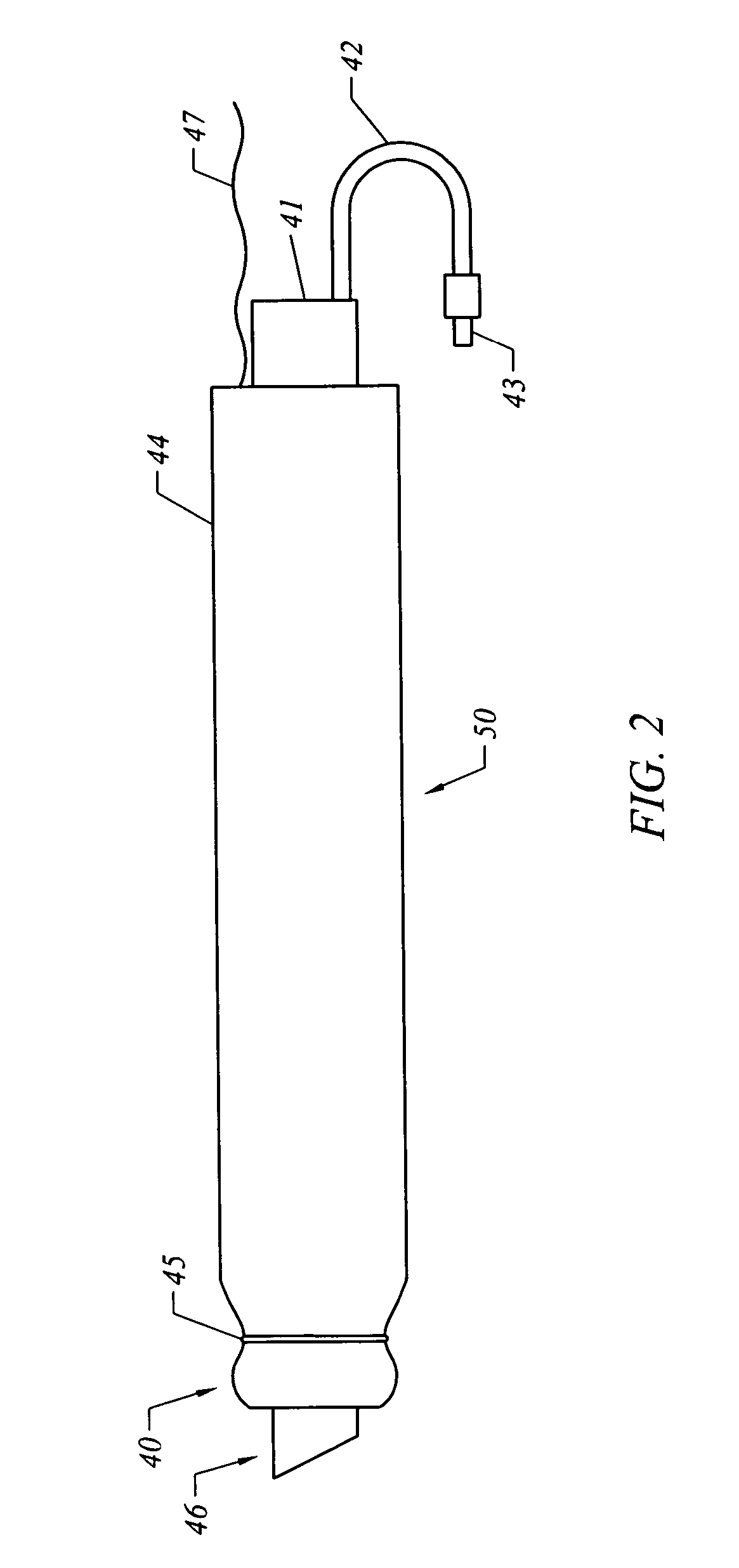
[0035] The present invention comprises systems, devices, and methods for the control of bleeding in body cavities, such as nasal passageways. Generally, the terms “cavity” and “passageway” may include any bodily cavity, recess, passageway, etc., other than a blood vessel or other component of the vasculature system, and it encompasses those which are healthy and normal as well as those which are abnormal and / or pathological (meaning, diseased or unhealthy).
[0036] The term “hemostatic” agent (or material) refers to any agent or material that is capable of arresting, stemming, or preventing bleeding by means other than inducing tissue growth alone. In other words, something other than tissue growth is at least partially responsible for retarding or preventing bleeding. Preferably, the agent or material will be one that enhances blot clot formation. It will, of course, be appreciated that the agent or material may have the beneficial property of inducing tissue growth in addition to r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com