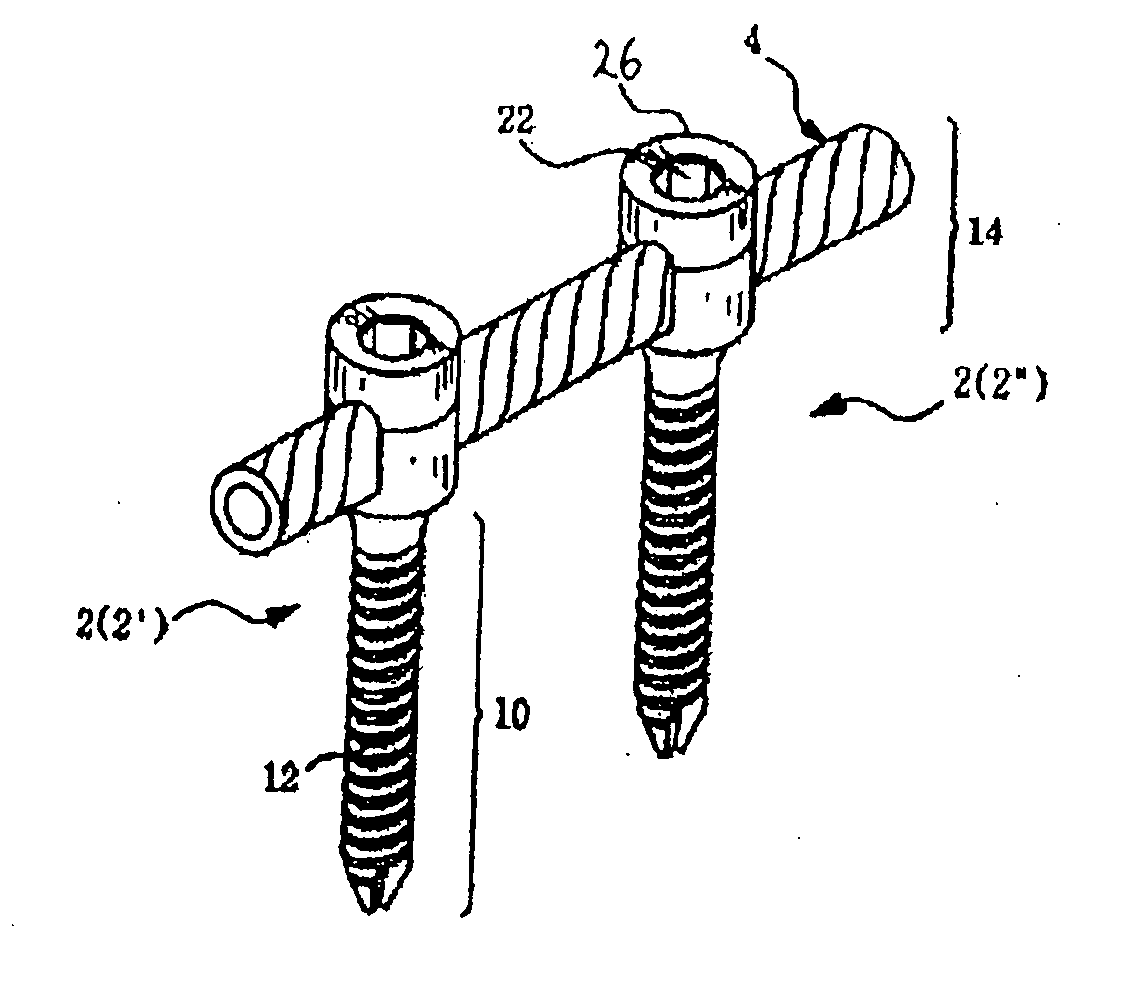
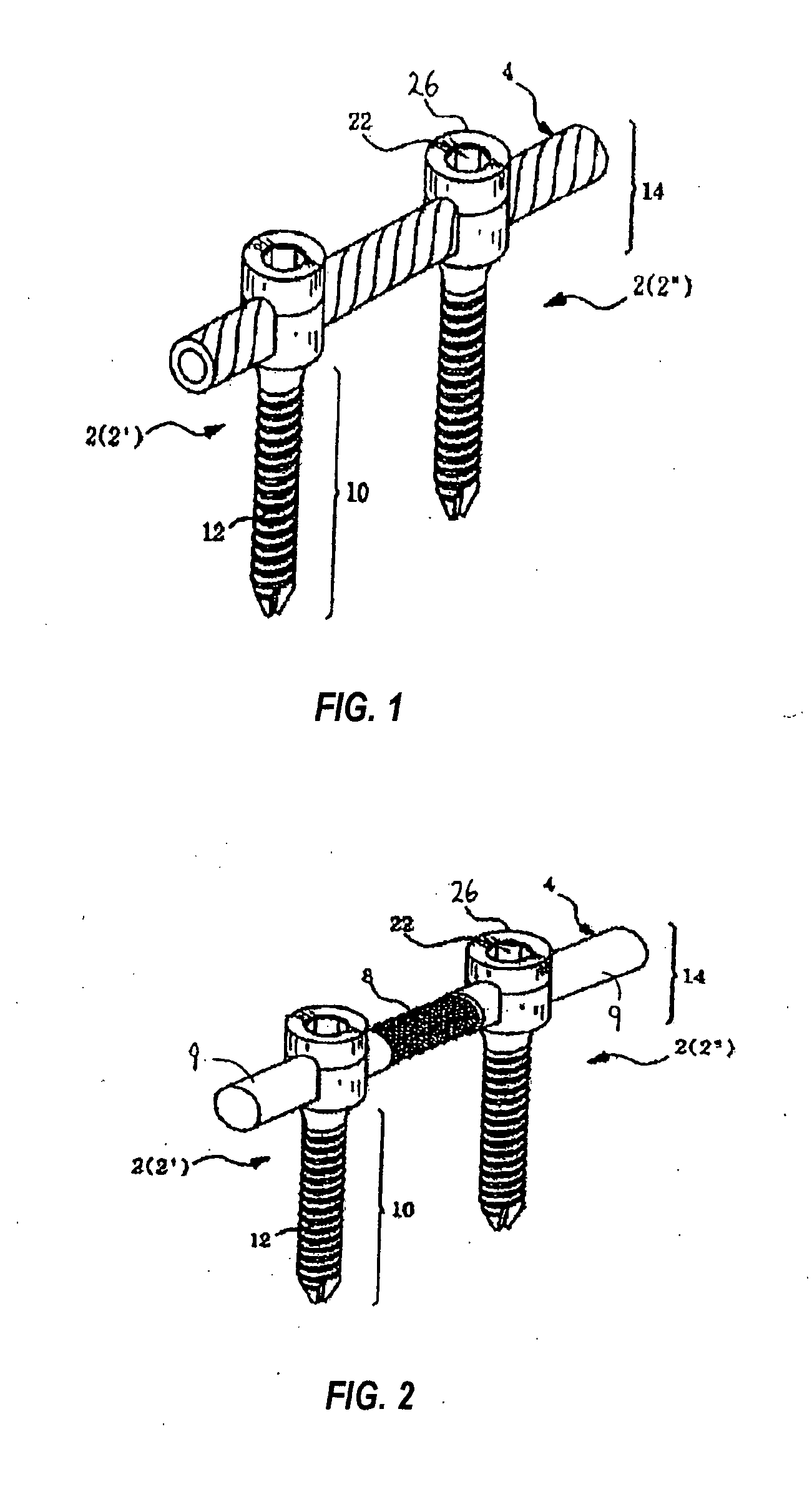
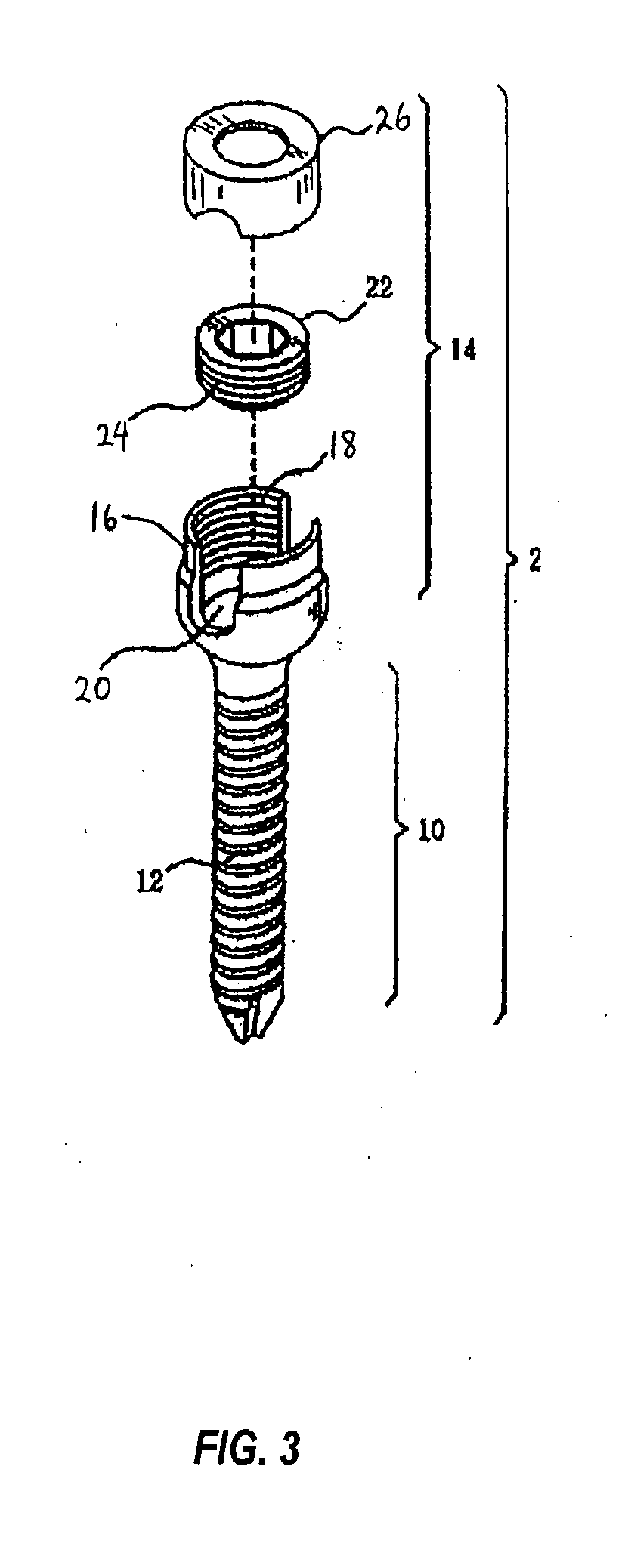
However, following the surgical procedure, fusion takes additional time to achieve maximum stability and a spinal fixation device is typically used to support the spinal column until a desired level of fusion is achieved.
However, because the connection units prevent normal movement of the spinal column, after prolonged use, the spinal fixation device can cause ill effects, such as “junctional syndrome” (transitional syndrome) or “fusion disease” resulting in further complications and abnormalities associated with the spinal column.
In particular, due to the high rigidity of the rods or plates used in conventional fixation devices, the patient's fixed joints are not allowed to move after the surgical operation, and the movement of the spinal joints located above or under the operated area is increased.
Consequently, such spinal fixation devices cause decreased mobility of the patient and increased stress and instability to the spinal column joints adjacent to the operated area.
It has been reported that excessive rigid spinal fixation is not helpful to the fusion process due to load shielding caused by rigid fixation.
However, because these devices are intended for use following a bone fusion procedure, they are not well-suited for spinal fixation without fusion.
Thus, in the end result, these devices do not prevent the problem of rigid fixation resulting from fusion.
Thus, it is effective in selected cases but is not appropriate for cases that require greater stability and fixation.
However, it has not yet been determined whether the Dynesys device can maintain long-term stability with flexibility and durability in a controlled study.
Because it has polyethylene components and interfaces, there is a risk of mechanical failure.
Furthermore, due to the mechanical configuration of the device, the surgical technique required to attach the device to the spinal column is complex and complicated.
These devices are flexible but they are not well-suited for enduring long-term axial loading and stress.
The design of existing flexible fixation devices are not well suited to provide varying levels of flexibility to provide optimum results for each individual candidate.
However, this patent is primarily concerned with providing a spinal fixation device that permits “relative longitudinal translational sliding movement along [the] vertical axis” of the spine and neither teaches nor suggests any particular designs of connection units (e.g., rods or plates) that can provide various flexibility characteristics.
Because they are typically very thin to provide suitable flexibility, such prior art rods are prone to mechanical failure and have been known to break after implantation in patients.
Therefore, conventional spinal fixation devices have not provided a comprehensive and balanced solution to the problems associated with curing spinal diseases.
Many of the prior devices are characterized by excessive rigidity, which leads to the problems discussed above while others, though providing some flexibility, are not well-adapted to provide long-term stability and / or varying degrees of flexibility.
Thus, in conventional spinal fixation procedures, the patient's back is incised about 10˜15 cm, and as a result, the back muscle, which is important for maintaining the spinal column, is incised or injured, resulting in significant post-operative pain to the patient and a slow recovery period.
One of the most challenging aspects of performing the minimally invasive spinal fixation procedure is locating the entry point for the pedicle screw under endoscopic or microscopic visualization.
Usually anatomical landmarks and / or radiographic devices are used to find the entry point, but clear anatomical relationships are often difficult to identify due to the confined working space.
The removal of this soft tissue results in bleeding in the affected area, thereby adding to the difficulty of finding the correct position to insert the securing members and causing damage to the muscles and soft tissue surrounding the surgical area.
Furthermore, because it is difficult to accurately locate the point of insertion for the securing members, conventional procedures are unnecessarily traumatic.
However, it is often difficult to obtain clear images required for finding the corresponding position of the spinal pedicle using radiography techniques due to radiographic interference caused by metallic tools and equipment used during the surgical operation.
Moreover, reading and interpreting radiographic images is a complex task requiring significant training and expertise.
Radiography poses a further problem in that the patient is exposed to significant amounts of radiation.
Although some guidance systems have been developed which guide the insertion of a pedicle screw to the desired entry point on the spinal pedicle, these prior systems have proven difficult to use and, furthermore, hinder the operation procedure.
Although the concept of the wire guidance system is a good one, in practice, the guide wire has been very difficult to use.
Because it is a relatively long and thin wire, the structural integrity of the guide wire often fails during attempts to drive one end of the wire into the pedicle bone, making the process unnecessarily time-consuming and laborious.
Furthermore, because the wire bends and crimps during insertion, it does not provide a smooth and secure anchor for guiding subsequent tooling and pedicle screws to the entry point on the pedicle.
Thus, current wire guidance systems pose a potential risk of misplacement or pedicle breakage.
Finally, because one end of the wire remains protruding out of the head of the pedicle screw, and the patient's back, this wire hinders freedom of motion by the surgeon in performing the various subsequent procedures involved in spinal fixation surgery.
Most conventional spinal fixation devices are too rigid and inflexible.
This excessive rigidity causes further abnormalities and diseases of the spine, as well as significant discomfort to the patient.
Although some existing spinal fixation devices do provide some level of flexibility, these devices are not designed or manufactured so that varying levels of flexibility may be easily obtained to provide a desired level of flexibility for each particular patient.
Additionally, prior art devices having flexible connection units (e.g., rods or plates) pose a greater risk of mechanical failure and do not provide long-term durability and stabilization of the spine.
Furthermore, existing methods of performing the spinal fixation procedure are unnecessarily traumatic to the patient due to the difficulty in finding the precise location of the spinal pedicle or sacral of the backbone where the spinal fixation device will be secured.
 Login to View More
Login to View More  Login to View More
Login to View More