Compositions Comprising Liposomes, An Antigen, A Polynucleotide and A Carrier Comprising a Continuous Phase of a Hydrophobic Substance
a technology of liposomes and antigens, which is applied in the direction of antibacterial agents, dsdna viruses, antibacterial ingredients, etc., can solve the problems of not teaching or suggesting combining antigens, and achieve the effect of increasing antibody titers and increasing the percentage of activated or memory cd8+
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0133]Pathogen free, female CD1 mice, 6-8 weeks of age, were obtained from Charles River Laboratories (St Constant, QC, Canada) and were housed according to institutional guidelines with water and food ad libitum, under filter controlled air circulation.
[0134]The H5N1 recombinant hemagglutinin protein, was purchased from Protein Sciences (Meridien, Conn., USA). This recombinant protein has an approximate molecular weight of 72,000 daltons and corresponds to the hemagglutinin glycoprotein, an antigenic protein present on the surface of the H5N1 influenza virus. This recombinant protein, hereafter designated rHA, was used as a model antigen to test the efficacy of vaccine formulations. rHA was used at 1 microgram per 30 microliter dose.
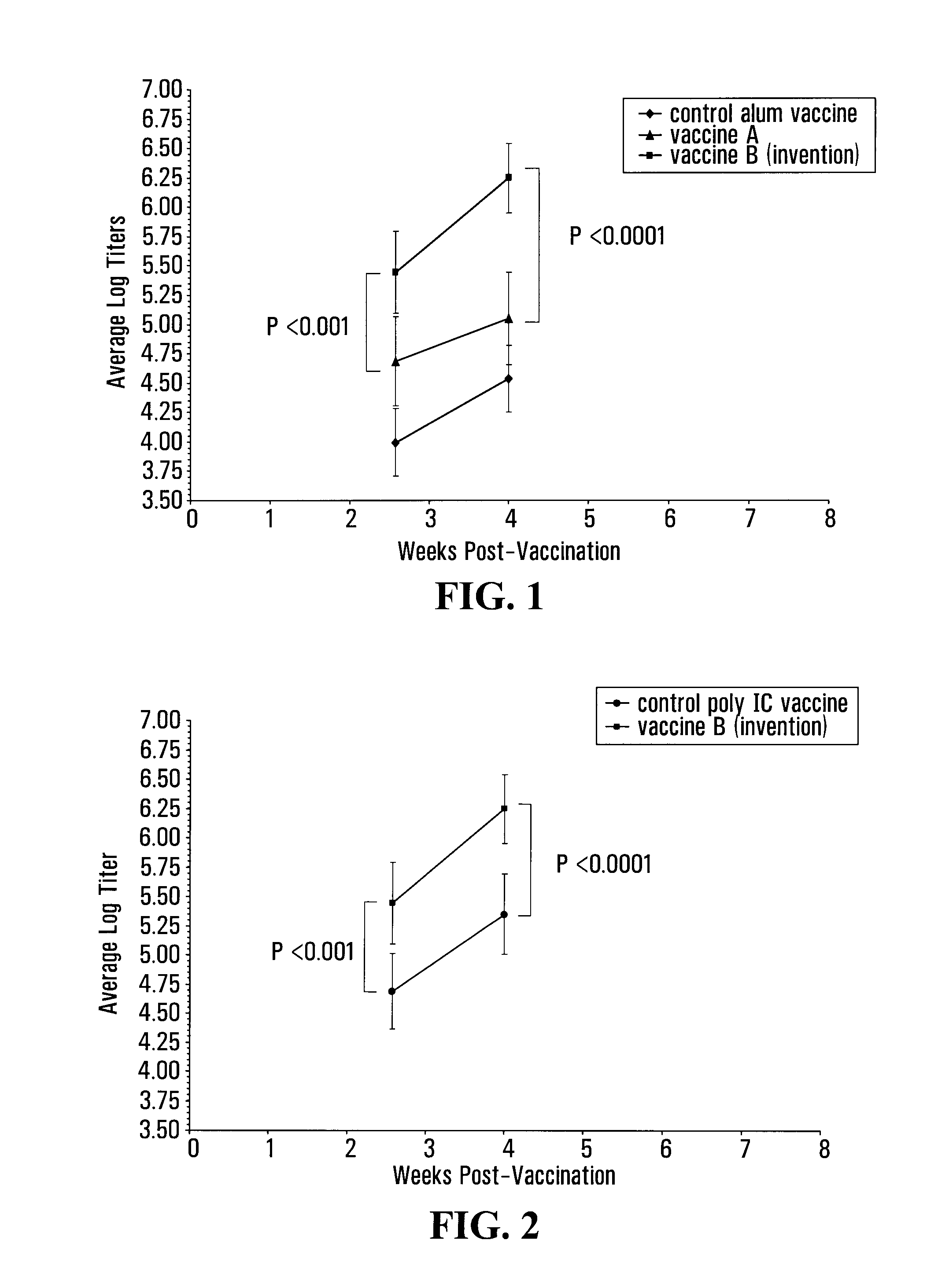
[0135]Vaccine efficacy was assessed by enzyme-linked immunosorbent assay (ELISA), a method that allows the detection of antigen-specific antibody levels in the serum of immunized animals. Performing the ELISA on sera collected from immunized mice on a r...
example 2
[0141]Pathogen free, female CD1 mice, 6-8 weeks of age, were obtained from Charles River Laboratories (St Constant, QC, Canada) and were housed according to institutional guidelines with water and food ad libitum, under filter controlled air circulation.
[0142]As in example 1, H5N1 recombinant hemagglutinin protein, corresponding to the hemagglutinin glycoprotein on the surface of the H5N1 influenza virus, was purchased from Protein Sciences (Meridien, Conn., USA). This recombinant protein, hereafter designated rHA, was used as a model antigen to test the efficacy of vaccine formulations. rHA was used at 1 microgram per 30 microliter dose.
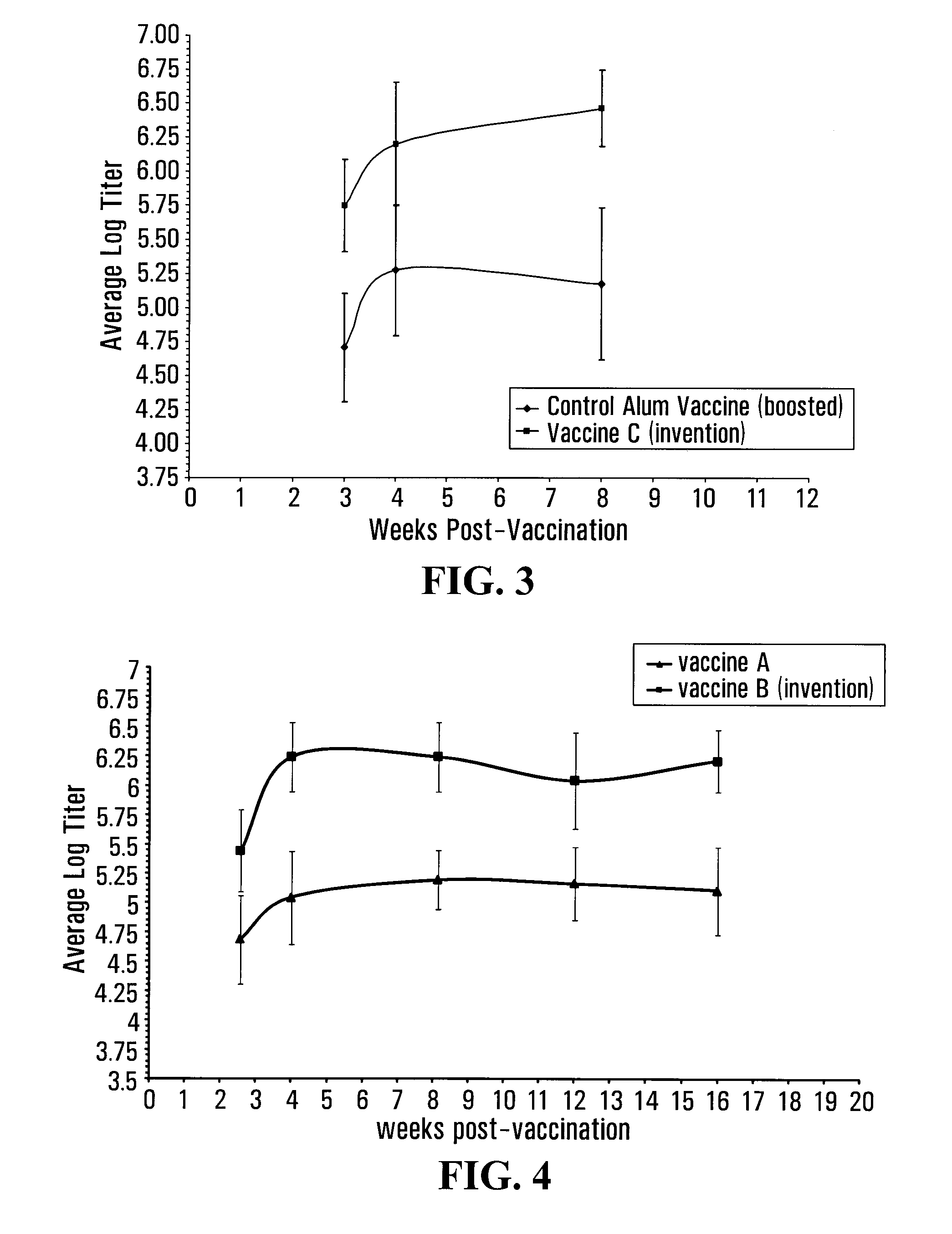
[0143]To formulate the vaccine corresponding to the invention, the same procedures as described in example one were used. In summary, 33 micrograms of rHA were suspended in 300 microliters of phosphate buffered saline (pH 7.4) then added to 132 milligrams of a S100 lecithin / cholesterol mixture (Lipoid GmbH, Germany) to form approximately 450 microli...
example 3
[0146]Pathogen free, female CD1 mice, 6-8 weeks of age, were obtained from Charles River Laboratories (St Constant, QC, Canada) and were housed according to institutional guidelines with water and food ad libitum, under filter controlled air circulation.
[0147]As in examples 1 and 2, H5N1 recombinant hemagglutinin protein, corresponding to the hemagglutinin glycoprotein on the surface of the H5N1 influenza virus, was purchased from Protein Sciences (Meridien, Conn., USA). This recombinant protein, hereafter designated rHA, was used as a model antigen to test the efficacy of vaccine formulations. rHA was used at 1 microgram per 50 microliter dose.
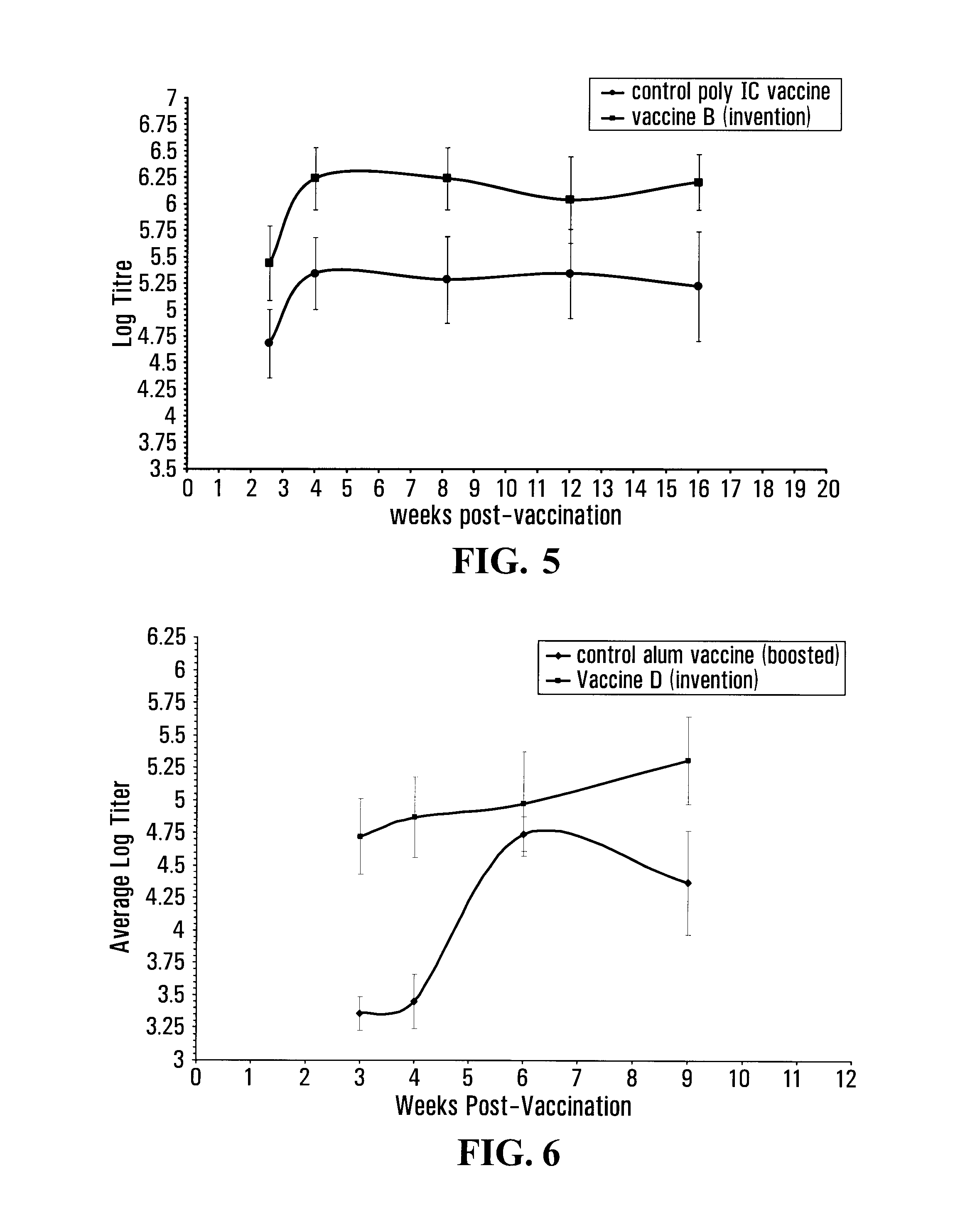
[0148]To formulate vaccine corresponding to the invention, a 10:1 w:w homogenous mixture of S100 lecithin and cholesterol (Lipoid GmbH, Germany) was hydrated in the presence of a rHA and polyI:C adjuvant (Pierce, Rockford, Ill., USA) solution in phosphate buffer to form liposomes with encapsulated rHA and adjuvant. In brief, 20 micrograms of ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap