Pharmaceutical blister
a technology for pharmaceuticals and blisters, applied in the field of pharmaceutical blisters, can solve the problems of changing the pharmaceutical quality of the drug stored therein, altering the pharmaceutical formulation, and blisters known from the prior art do not necessarily adequately protect the formulation embedded therein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
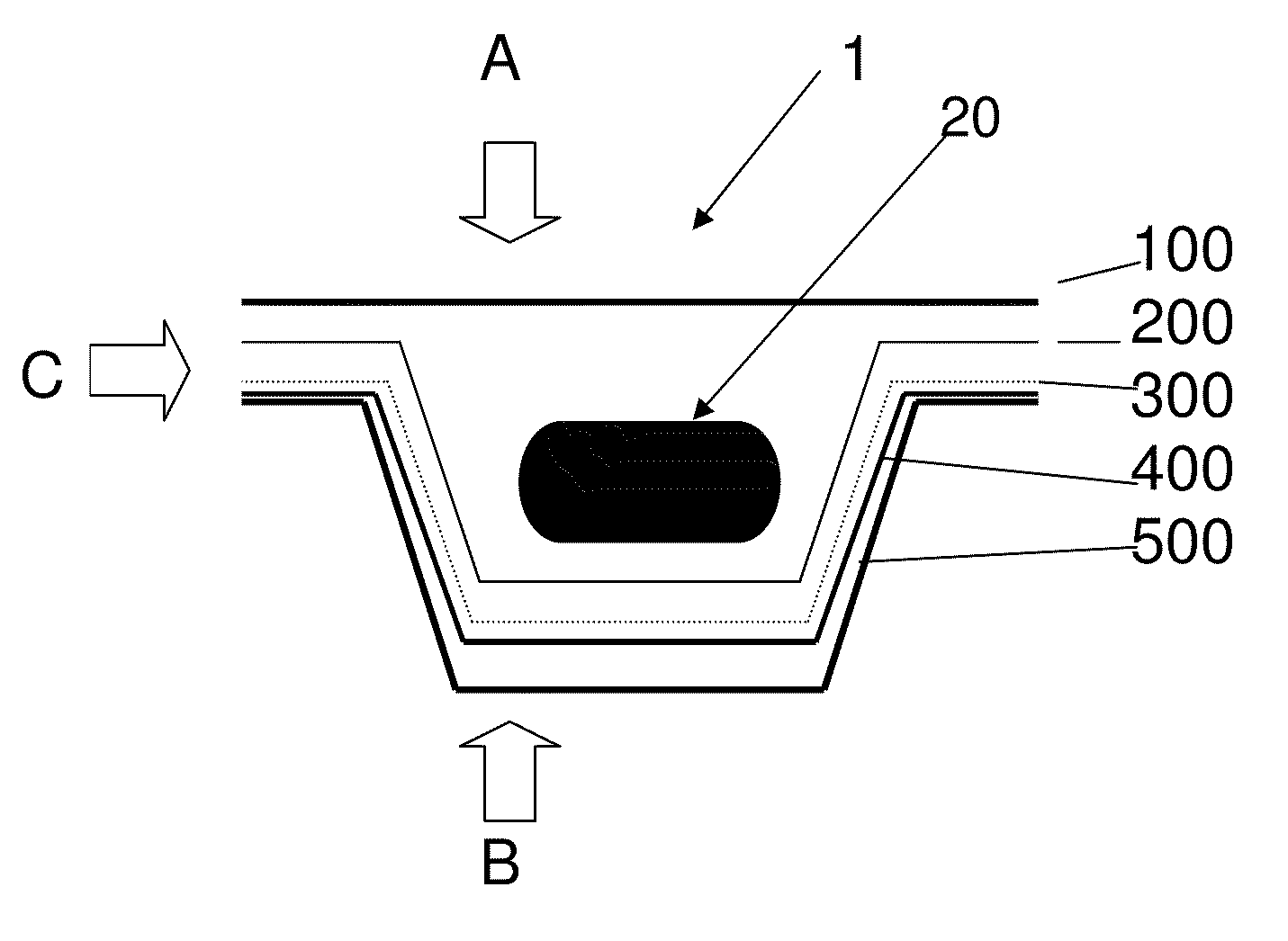
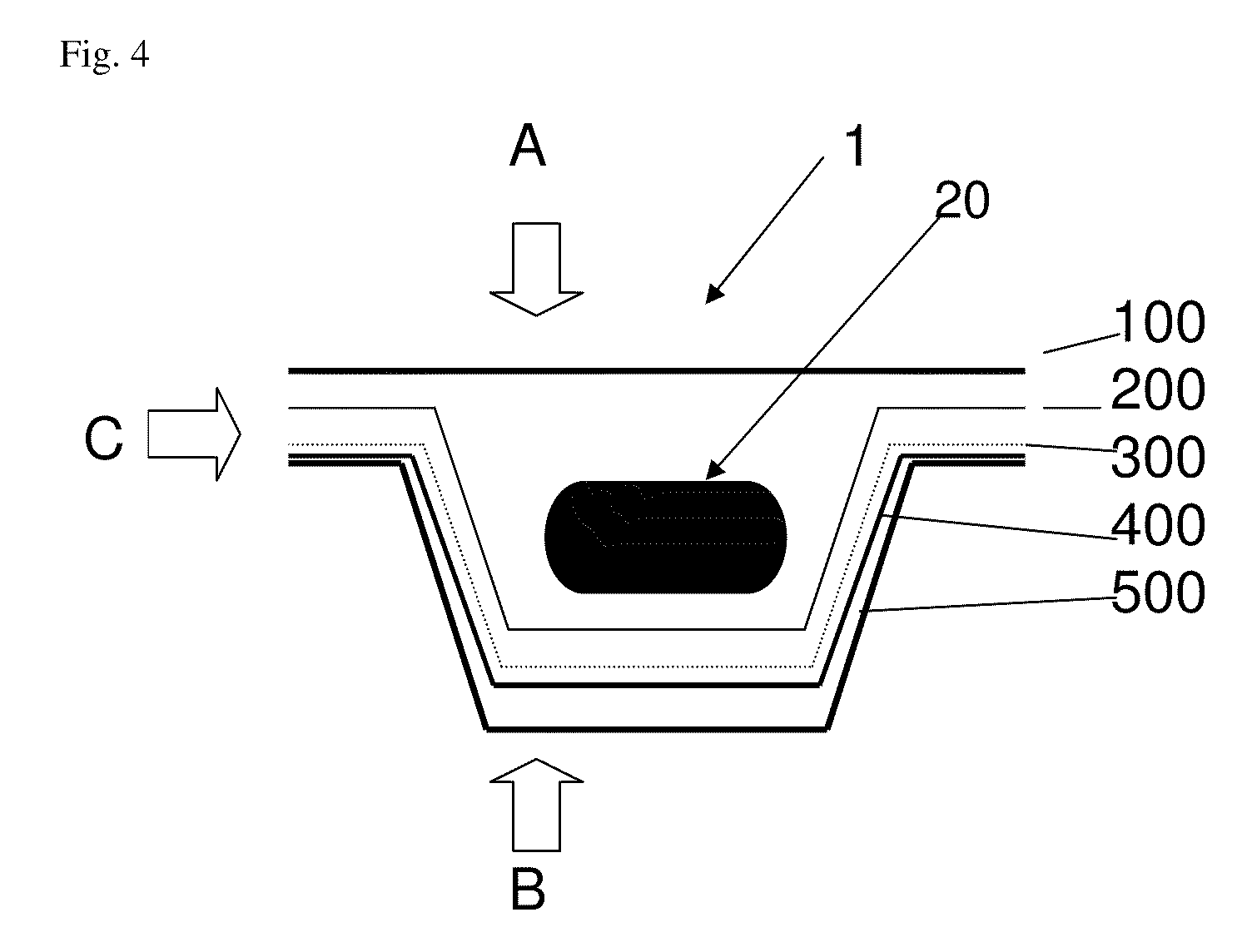
[0011]An aim of the invention is therefore to provide a transparent, flexible, sealable blister[0012]1) with improved protection against gas and moisture exchange between the inside of the blister and the outer environment,[0013]2) with sufficient transparency / translucency for visual inspection and[0014]3) sufficient mechanical stability so as not to peel off when the blister is bent or used.
[0015]The disadvantages known from the prior art should also be eliminated.
DETAILED DESCRIPTION OF THE INVENTION
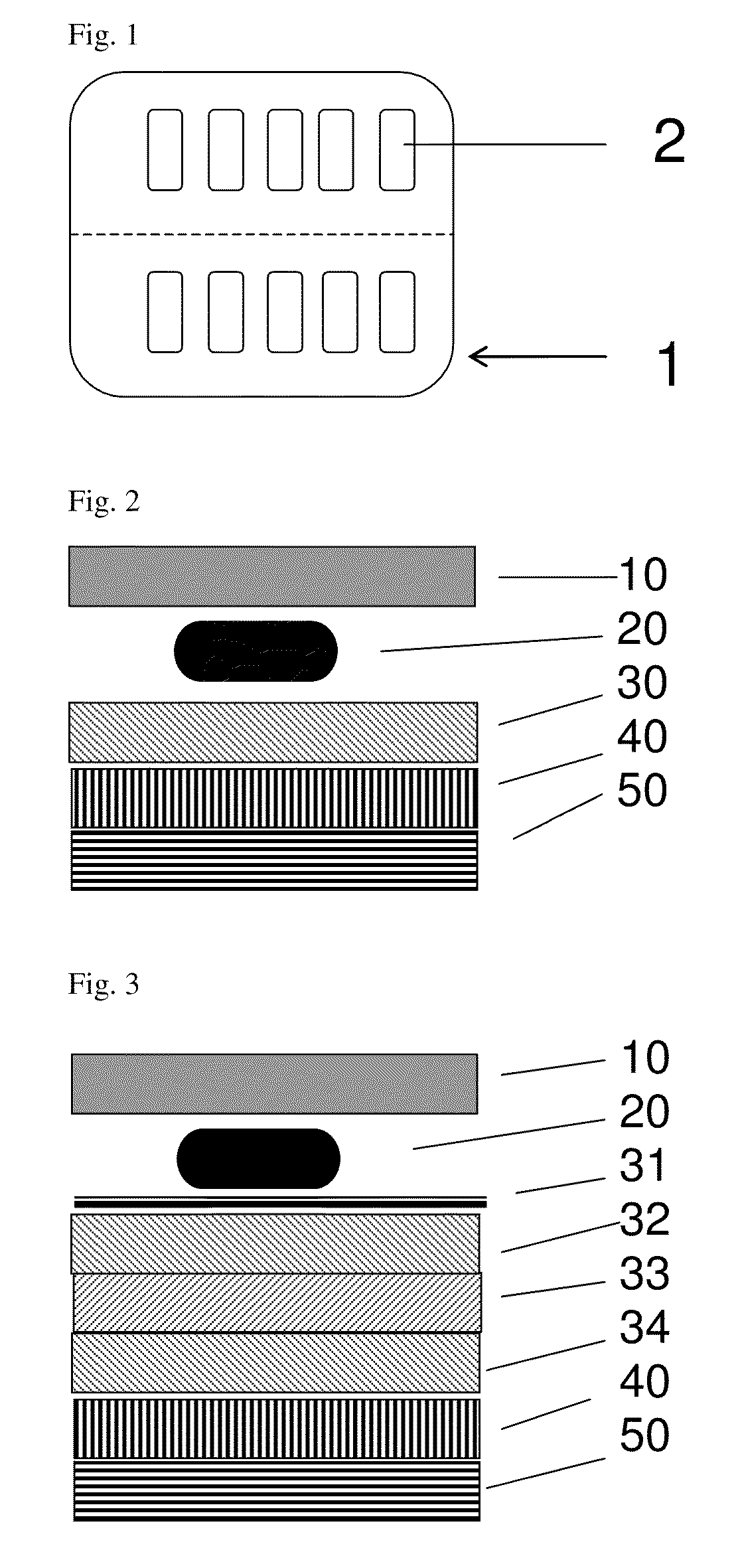
[0016]It is proposed according to the invention to coat the base and / or cover foil of a pharmaceutical blister consisting of plastics with an additional functional layer containing silicon oxide and carbon, so as to reduce the above-mentioned gas permeability of the actual blister.
[0017]If a foil of the blister (preferably the cover foil) consists of aluminum, it is sufficient to coat the preferably transparent or translucent plastics foil (preferably the base foil, as plastics foils c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com