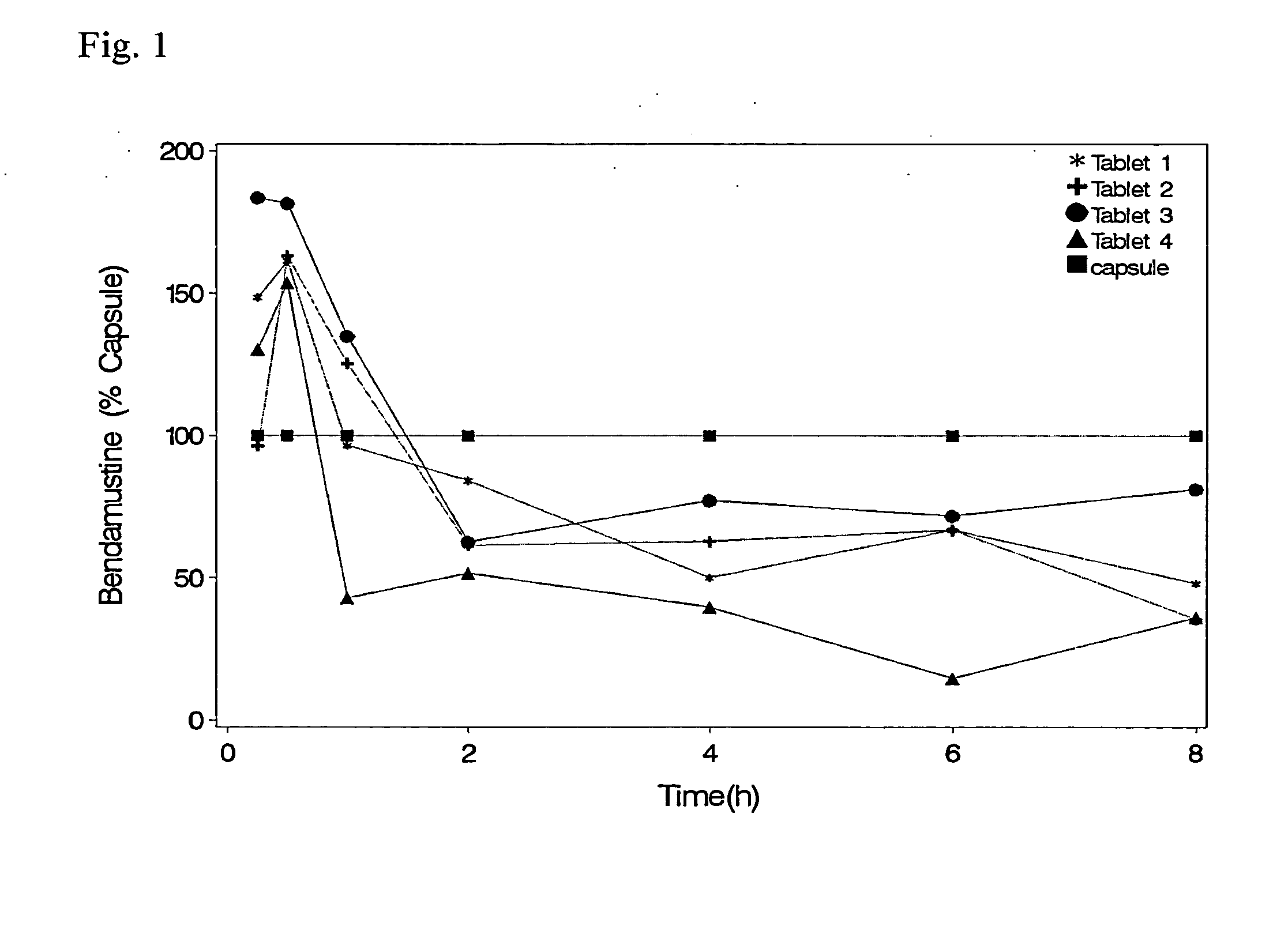
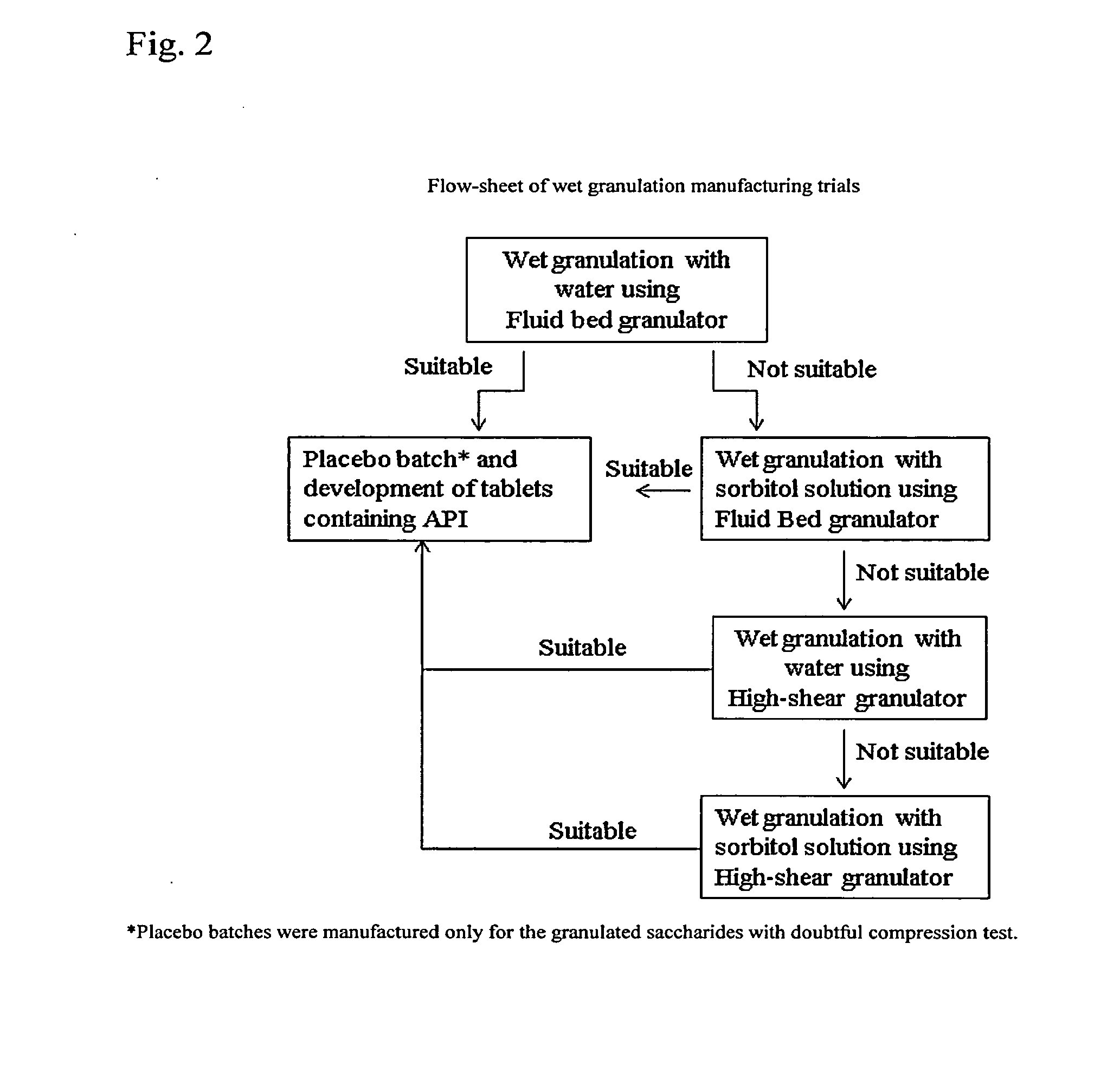
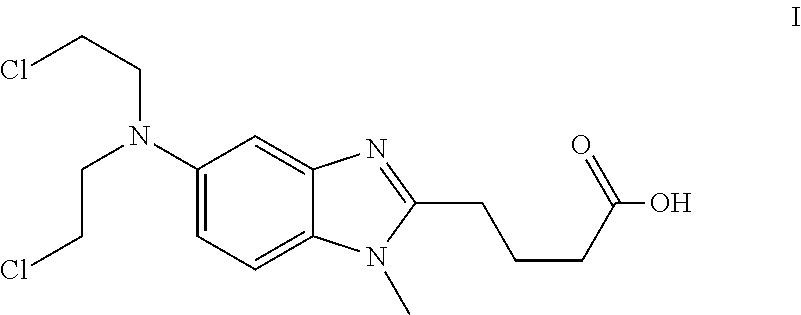
Solid Dosage Forms of Bendamustine
a technology of bendamustine and solid dosage forms, which is applied in the direction of drug compositions, immunological disorders, microcapsules, etc., can solve the problems of time-consuming, burdensome, and difficult reconstitution for healthcare professionals, and achieve the effect of improving the dissolution profil
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1a
[0060]For compatibility testing mixtures containing bendamustine hydrochloride and an excipient at a ratio of 1:1 (m / m) were prepared. The excipients were selected from mannitol and lactose. After preparation the mixtures were packed in clear glass HPLC-Vials (6 ml) Agilent and stored at different storage conditions as shown in Table 1 below. At defined time points samples were removed from storage and tested for purity (HPLC; column: Zorbax Bonus-RP, 5 μm; temperature of column oven: 30° C.; temperature of autosampler: 5° C.; detector: 254 nm) and appearance.
TABLE 1Storage ConditionsBendamustine hydrochloride and excipients for oral formulationTested time pointsT = 1Storage conditionT = 0month(1) 50° C., Vials closedn = 2n = 1(2) 70° C., Vials closed*n = 2n = 2(3) 40° C. / 75% r.h., Vials open**n = 2n = 2*stored at 50° C. for one month before storage at 70° C.**stored at 25° C. / 60% r.h. for one month before storage at 40° C. / 75%
[0061]In all these mixtures, the bendamustine hydrochlor...
example 1b
[0063]For further compatibility testing in accordance with the methods of example 1a, mixtures containing bendamustine hydrochloride and an excipient at a ratio of 1:1 (m / m) were prepared. The excipients were selected from Opadry®, Eudragit® E PO, sodium carboxymethylcellulose (Avicel® RC 591) and cross-linked polyvinylpyrrolidone (Crospovidone).
[0064]In the case of Eudragit® E PO the initial amounts of the impurities HP1 (hydrolysis product) and BM1DIMER were significantly increased (HP 1: 1.5%, BM1DIMER: 1%) but during storage a decrease of these impurities could be detected at all storage conditions independent of the influence of humidity. In the case of cross-linked polyvinylpyrrolidone a significant increase of HP 1 from 0.1% to 0.4% could be detected at the storage condition 40° / 75% R.H. / vials open. At all other storage conditions (vials closed) no increase of HP1 could be detected.
[0065]The appearance of the mixtures containing Eudragit® E PO and cross-linked polyvinylpyrrol...
example 2
[0067]253 g of a mixture comprising mannitol as the main excipient and microcrystalline cellulose, Ac-Di-Sol®, colloidal silicon dioxide, talc and stearic acid in the relative quantities mentioned in the following table 2a was prepared by mixing in a 1 liter cube blender (Erweka) for 15 minutes. Thereafter 10.612 g of the mixture and 3.0 g of bendamustine hydrochloride were sieved through a 0.425 mm sieve and then transferred into a Turbula mixer T2A, equipped with a glass vial of 50 ml and subsequently mixed for 10 minutes at 60 rpm.
[0068]From this mixture round tablets were compressed having the following characteristics:
[0069]Mean value diameter: 9.1 mm; mean value mass: 247.7 mg; mean value hardness: 81N.
TABLE 2aTabletComponentmg / dosage-formRelative Content %bendamustine hydrochloride55.122.04Mannitol141.456.56Microcrystalline cellulose25.010.00(Avicel ® PH112)Ac-Di-Sol ®12.55.00Colloidal silicon dioxide1.00.40(Aerosil ® 200)Talc12.55.00Stearic acid2.51.00
[0070]Tablets were stor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com