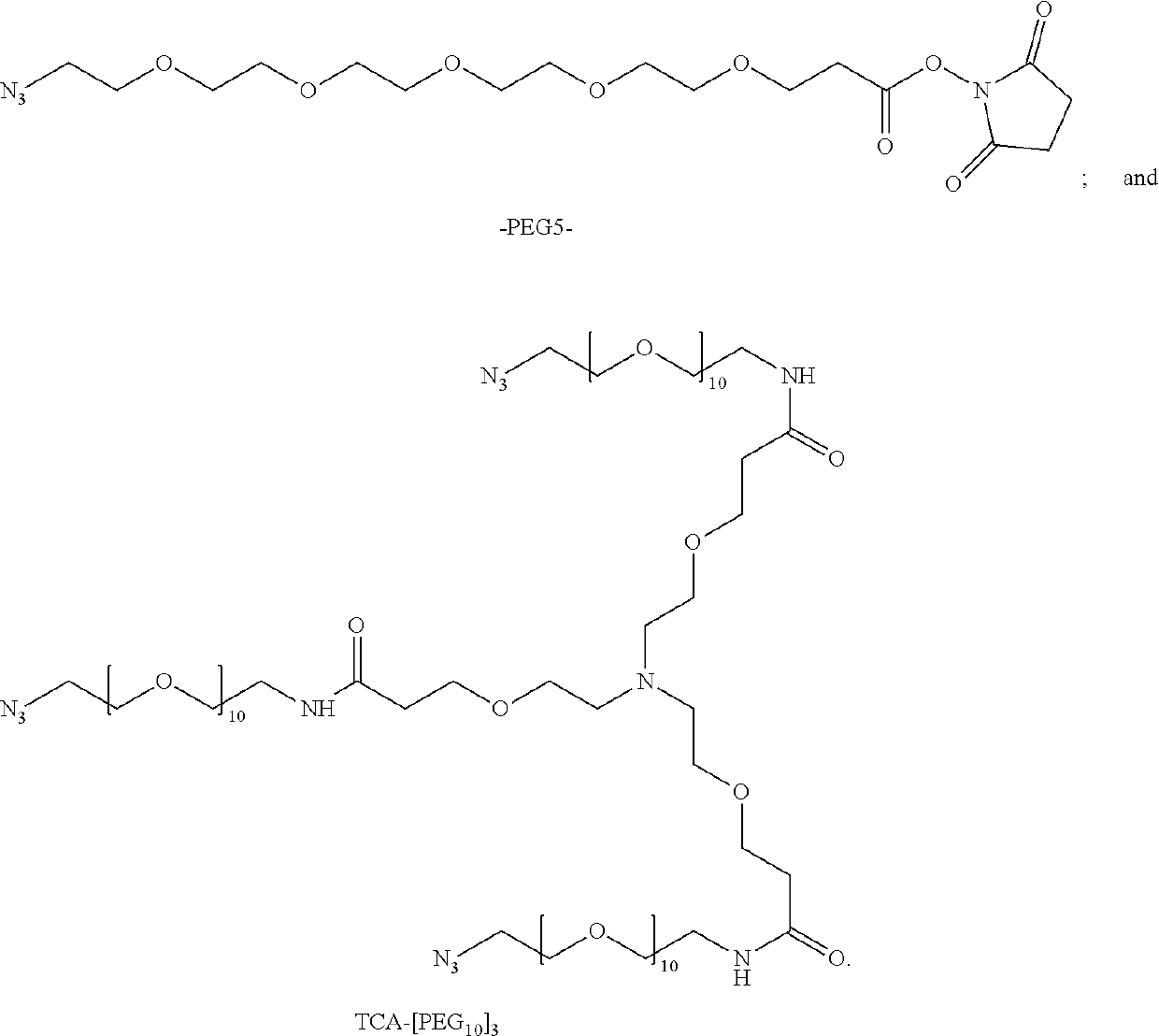
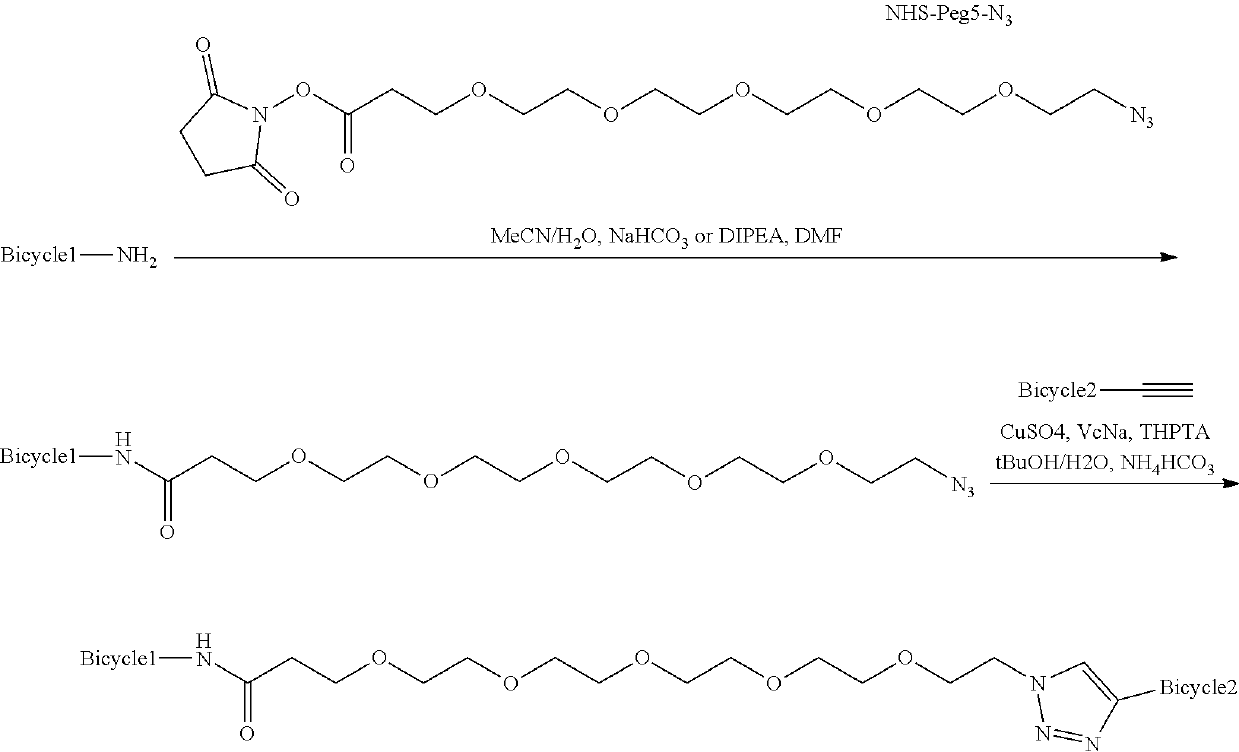
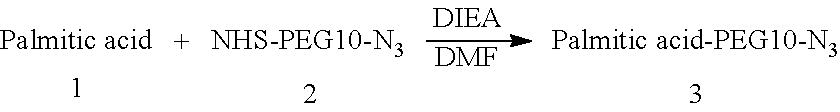Heterotandem bicyclic peptide complexes
a bicyclic peptide and complex technology, applied in the direction of peptides, drug compositions, peptides/protein ingredients, etc., can solve the problem of reducing the conformational flexibility of cyclic structures, and achieve the effect of preventing, suppressing or treating cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
of BCY12375
[0162]
[0163]Procedure for Preparation of Palmitic Acid—PEG10-N3
[0164]A mixture of Palmitic acid (100.0 mg, 282.89 μmol, 1.0 eq.), compound 2 (150.0 mg, 284.84 μmol, 1.0 eq.), and DIEA (74.5 mg, 574.11 μmol, 100.0 μL, 2.0 eq.) was dissolved in DMF (2 mL). The reaction mixture was stirred at 30° C. for 2 hr. LC-MS showed compound 1 was consumed completely and one main peak with desired m / z (MW: 765.03, observed m / z: 765.22) was detected. The reaction mixture was concentrated under reduced pressure to remove solvent and produced a residue. The residue was then purified by prep-HPLC (neutral condition). Palmitic acid—PEG10-N3 (79.0 mg, 99.41 μmol, 35.14% yield, 96.27% purity) was obtained as a white solid.
[0165]Procedure for Preparation of Palmitic Acid—PEG10-BCY12023
[0166]A mixture of compound 3 (50.0 mg, 22.07 μmol, 1.0 eq.), compound 2 (17.0 mg, 22.22 μmol, 1.0 eq.), and THPTA (10.0 mg, 23.02 μmol, 1.0 eq.) was dissolved in t-BuOH / H2O (1:1, 1 mL, pre-degassed and purged wi...
example 2
of BCY12021
[0171]
[0172]Procedure for Preparation of Palmitic Acid—PEG10-BCY11144
[0173]A mixture of compound 3 (160.0 mg, 69.45 μmol, 1.0 eq.), compound 4 (56.0 mg, 72.20 μmol, 1.0 eq.), and THPTA (35.0 mg, 80.55 μmol, 1.1 eq.) was dissolved in t-BuOH / H2O (1:1, 2 mL, pre-degassed and purged with N2 for 3 times), and then CuSO4 (0.4 M, 56.0 μL, 1.0 eq.) and VcNa (30.0 mg, 151.43 μmol, 2.2 eq.) were added under N2. The pH of this solution was adjusted to 8 by dropwise addition of 0.2 M NH4HCO3 (in 1:1 t-BuOH / H2O), and the solution turned to light yellow. The reaction mixture was stirred at 40° C. for 16 hr under N2 atmosphere. LC-MS showed one main peak with desired m / z (calculated MW: 3068.70, observed m / z: 1533.81 ([M / 2+H]+), 1023.43 ([M / 3+H]+)) was detected. The reaction mixture was filtered and concentrated under reduced pressure to give a residue. The crude product was purified by prep-HPLC (TFA condition), and Palmitic acid—PEG10-BCY11144 (150.0 mg, 46.83 μmol, 67.42% yield, 95.8...
example 3
of BCY11468
[0178]
[0179]Procedure for Preparation of COM113
[0180]A mixture of compound 1 (50.0 mg, 124.4 μmol, 1.0 eq), EDCl (95.4 mg, 497.7 μmol, 4.0 eq), HOBt (55.5 mg, 410.6 μmol, 3.3 eq), and DMAP (15.2 mg, 124.4 μmol, 1.0 eq) was dissolved in 2 mL DMF, and then DIEA (134.9 mg, 1.04 mmol, 181.8 μL, 8.4 eq) was added to generate a homogenous solution. Next, compound 2 (200.0 mg, 379.8 μmol, 3.05 eq) dissolved in DMF (2 mL) was added to this solution dropwise. The reaction mixture was stirred at 30° C. for 16 hr. LC-MS showed compound 1 was consumed completely and one main peak with desired m / z (MW: 1891.19, observed m / z: 945.8600 ([M / 2+H+]) and 612.4400 ([(M−3H2O) / 3+H+])) was detected. The reaction mixture was directly purified by prep-HPLC (TFA condition), resulting in COM113 (161 mg, 85.67 μmol, 68% yield) as a yellow oil after lyophilization.
[0181]Procedure for Preparation of COM113-BCY8928
[0182]COM113 (50.0 mg, 26.44 μmol, 1.0 eq) and BCY8928 (53.0 mg, 23.9 μmol, 0.9 eq) were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com