Method for treating benign prostatic hyperplasia
a benign prostatic hyperplasia and treatment method technology, applied in the field of benign prostatic hyperplasia treatment, can solve the problems of its natural ability to induce hypercalcemia and hyperphosphatemia, and achieve the effects of reducing prostate size, preventing and/or treating bladder dysfunction, and reducing prostate siz
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
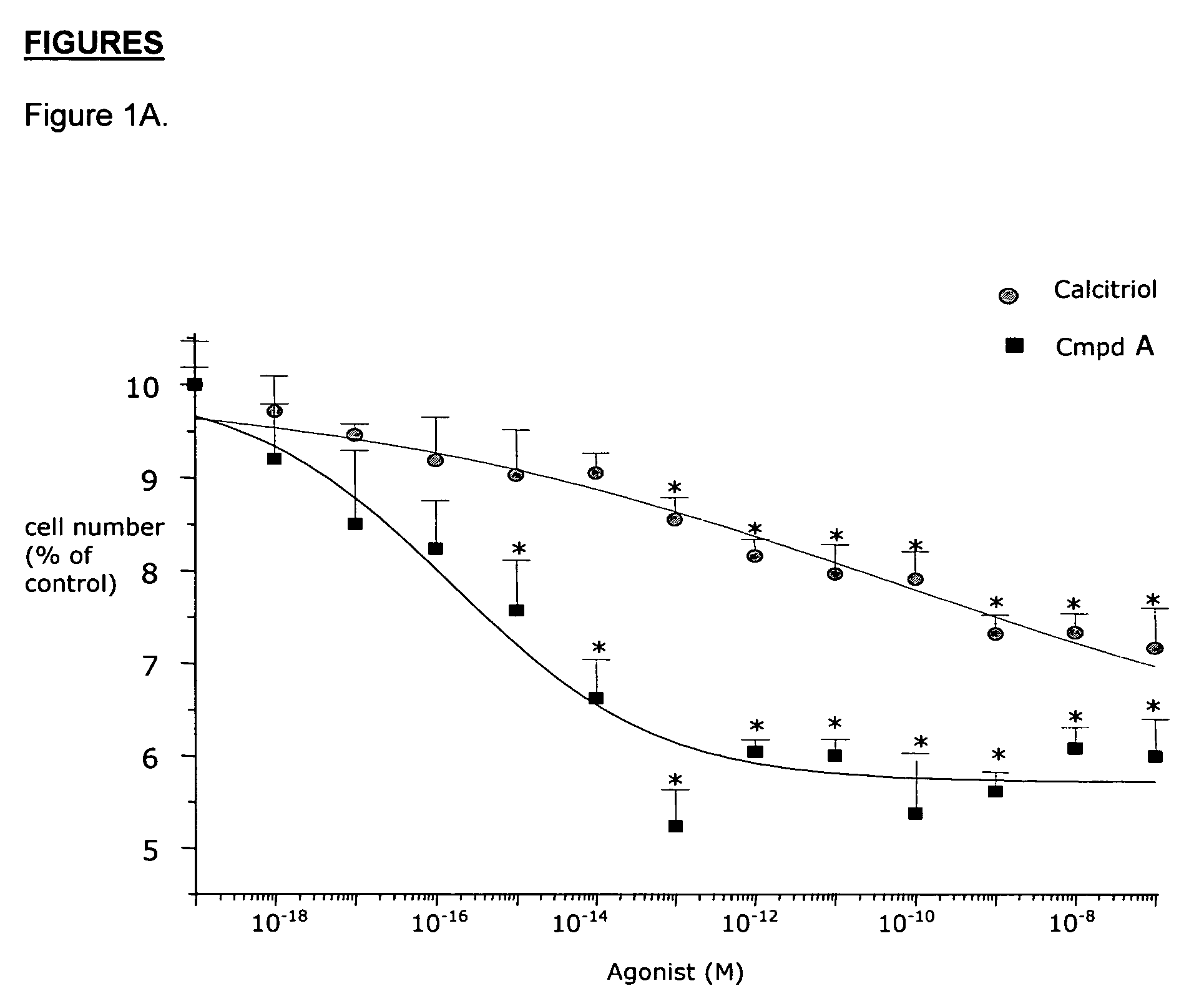
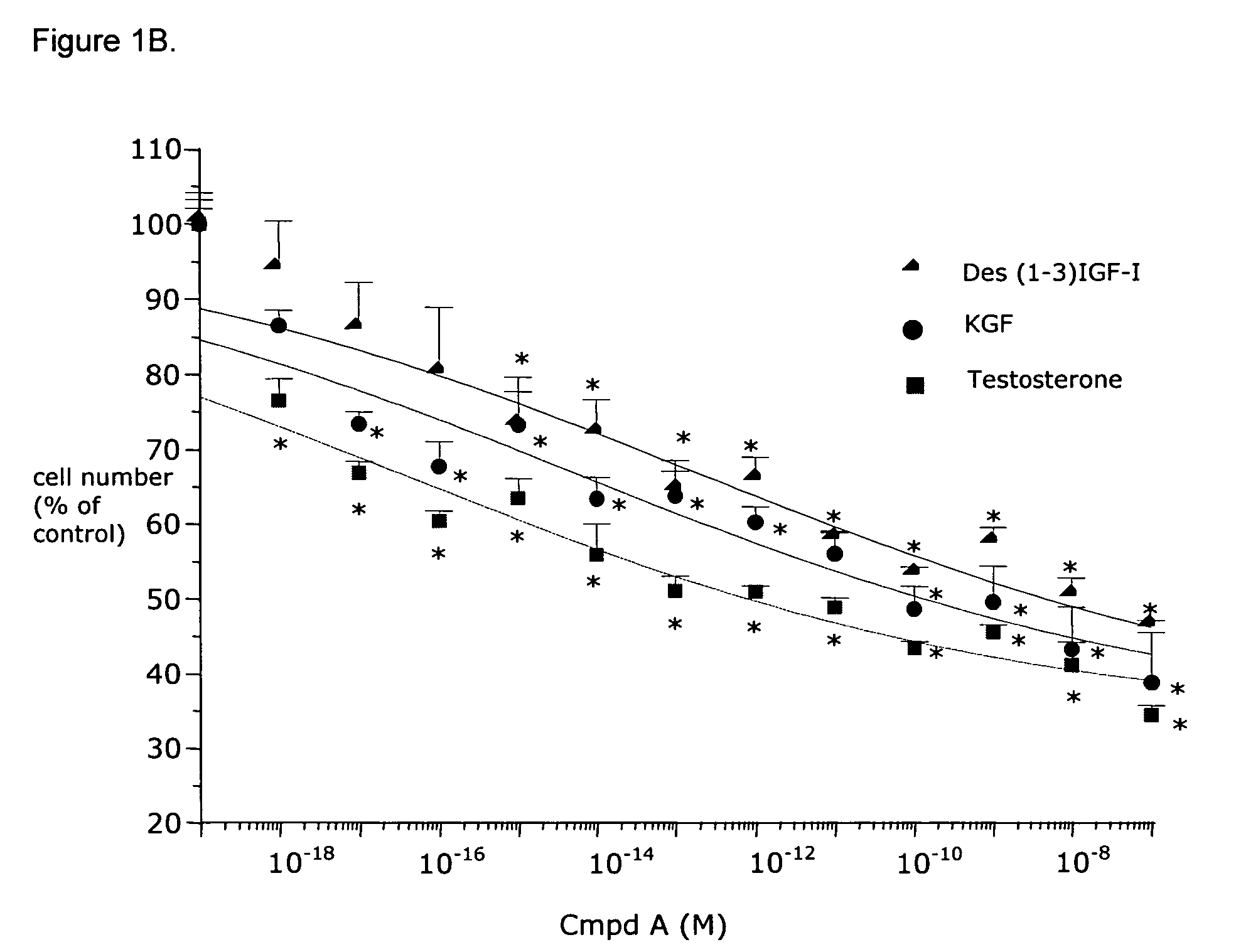
Effects of Compound A on BPH Cells in vitro
Material and Methods
Materials
[0081]Minimum Essential Medium (MEM), DMEM-F12 1:1 mixture, Ham's F12 medium, phosphate buffered saline (PBS), bovine serum albumin (BSA) fraction V, glutamine, geneticine, collagenase type IV, vitamin D3, testosterone (T), dihydrotestosterone (DHT), cyproterone acetate, β-nicotinamide adenine dinucleotide 3′-phosphate reduced form (NADPH), dithithreitol (DTT), phenylmethylsulfonyl fluoride (PMSF) and a kit for measuring calcemia were purchased from Sigma (St. Louis, Mo.). The protein measurement kit was from Bio-Rad Laboratories, Inc. (Hercules, Calif.). Fetal bovine serum (FBS) was purchased from Unipath (Bedford, UK). Monoclonal anti-rat clusterin antibody (mouse monoclonal IgG) specific for beta-chain was from UPSTATE Biotechnology (Lake Placid, N.Y.). Apop Tag kit for in situ end labelling (ISEL) was from Oncor (MD, USA). CHO 1827 and CHO 1829 were provided by Serono International (Geneva, Switzerland). Ins...
example 2
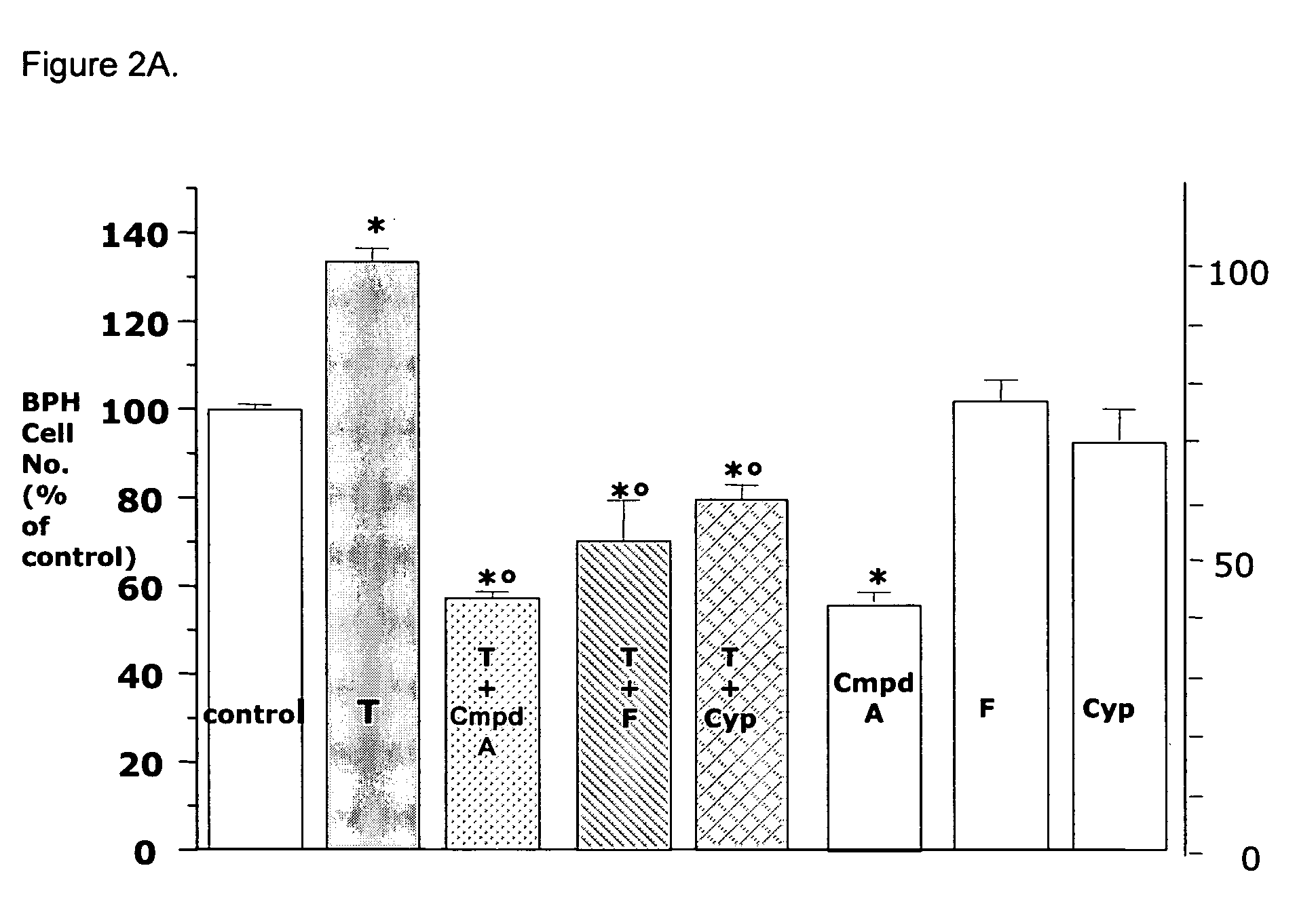
Anti-proliferative Properties of Compound A in in vivo Models of Prostate Growth
Animal Protocols
[0102]Male Sprague Dawley rats (28 days old) were purchased from Charles River Laboratories (Calco, Lecco, Italy). All animal experimentation described was conducted in accord with accepted standards of animal care. Castration was performed via the scrotal route under ketamine / xylazine anaesthesia. Three days after castration, rats (5-8 animals per group) were treated or not with T enanthate (30 mg / Kg) in two separate weekly sc injections. Rats were orally treated for 5 days the first week, and 4 days the second week with vehicle (Miyglyol 812), Compound A (10, 30, 100 and 300 μg / Kg) or finasteride (10 and 40 mg / Kg) for a total of 9 administrations, and sacrificed one day later.
[0103]Alternatively, intact, adult male Sprague Dawley rats (weight 250 g) were dosed orally with vehicle (Miglyol 812), Compound A (10, 30, 100 and 300 μg / Kg) or finasteride (10 and 40 mg / Kg) 5 days / week for 5 con...
example 3
Reduction of Prostate Weight in Healthy Dogs Treated with Compound A
[0120]A 9-month toxicity study was carried out in four groups of male beagle dogs, which were treated by daily oral gavage with 0.5 μg, 1.5 μg and 5 μg / kg body weight / day of Compound A (in vehicle Miglyol 812) or with vehicle alone. This treatment was followed by a 2-month recovery period for the group receiving the highest dose, 5 μg, after which prostate weights was measured. In addition to entirely favourable toxicity data, a lower prostate weight was observed at the end of treatment with Compound A (see FIG. 8) and after recovery (see FIG. 9). The results after recovery were analysed statistically via a one-tailed Student's t test and were found to be significantly different between Compound A and vehicle (P<0.05). These results further demonstrate the ability of Compound A to reduce prostate size in vivo.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com