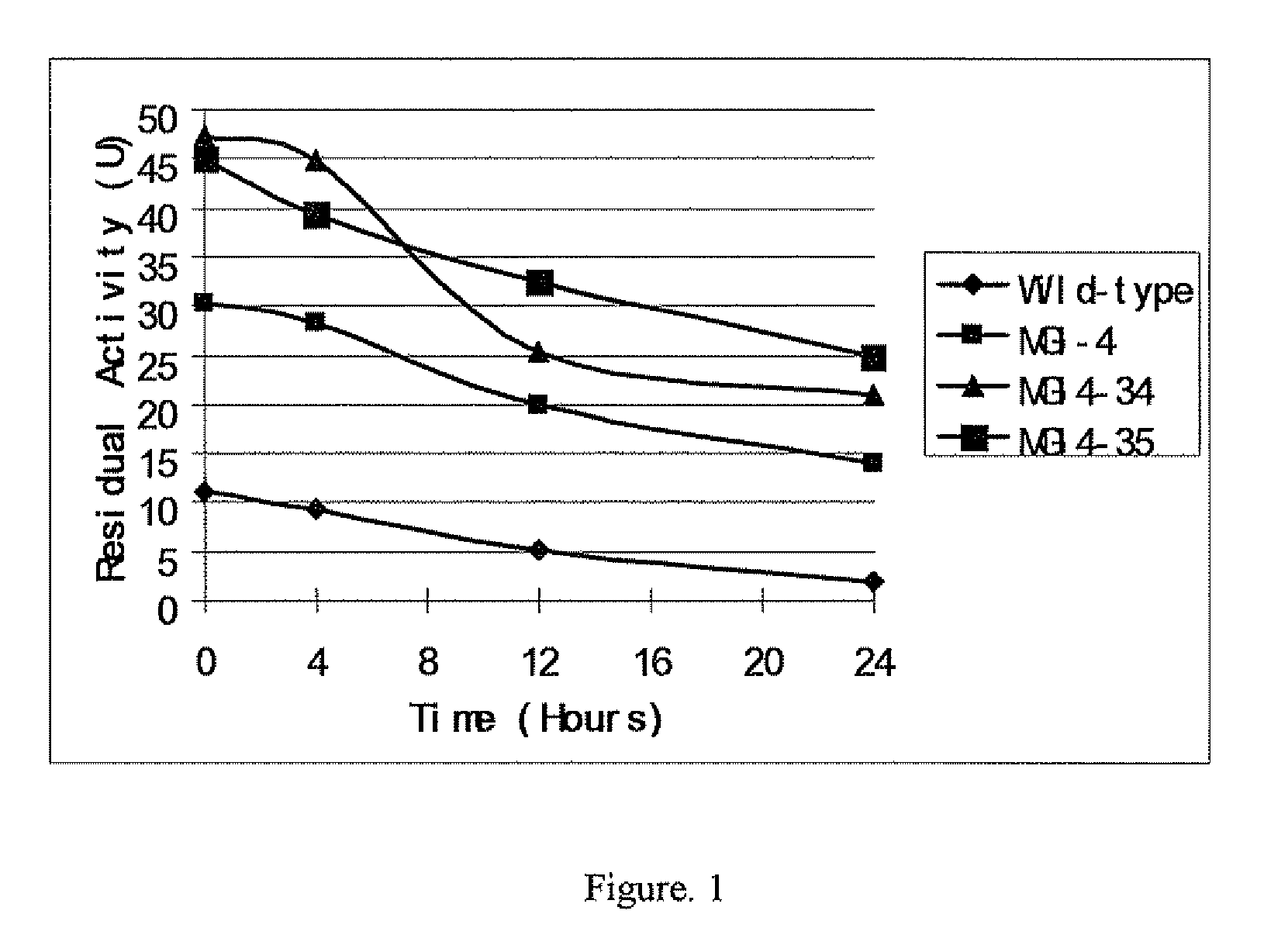
Method of making bioethanol by using glucose isomerase mutants
a technology of glucose isomerase and mutants, applied in biofuels, organic chemistry, enzymology, etc., can solve problems that are not applicable in industrial applications, and achieve the effect of improving catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Amplification of Wild-Type Glucose Isomerase and Construction of pGEMT-TS
[0021]Primers T1 and T2 (Table 1) were designed based on the sequence of GenBank L09699 and used to amplify the wild-type glucose isomerase gene from T. saccharolyticum ATCC 49915 (ATCC, USA).
[0022]The amplification condition was: 20 mM Tris-HCl (pH 8.8), 10 mM KCl, 10 mM (NH4)2SO4, 2 mM MgSO4, 0.1% Triton X-100, 50 μM dATP, 50 μM dTTP, 50 μM dCTP, 50 μM dGTP, 400 nM primer T1, 400 nM primer T2, 1.5 U Taq DNA polymerase (Promega, USA), a loopful of T. saccharolyticum colony, and the total volume was adjusted to 50 μl with sterile distilled water.
[0023]The PCR amplification program for the reaction was: 95° C., 3 min; then 40 cycles of 95° C., 50 sec, 50° C., 30 sec, 72° C., 1 min; and finally 72° C., 10 min. The amplified PCR product, about 1.5 kb in length, was ligated into vector pGEMT-Easy to generate pGEMT-TS. The pGEMT-TS was sequenced to determine the DNA sequence of the wild-type glucose isomerase as Seq...
example 2
Site-Directed Mutagenesis of Trp139 of Wild-Type Glucose Isomerase
[0024]The site directed mutagenesis was done as described by Ho et al., Gene 77:51-59, 1989 and White et al., PCR protocol: current methods and applications. Totowa, N.J.: Humana Press 1993.
[0025]With pGEMT-TS (Example 1) as template, the Trp (W) at position 139 of the wild-type glucose isomerase was mutated to Phe (F) to generate glucose isomerase mutant MGI-W139F by PCR amplification using primers 139FF and 139FR (Table 1) and universal primers T1 and T2 (Example 1).
[0026]Fragment T1FR was amplified using primer pair T1 and 139FR. Fragment FFT2 was amplified using primer pair 139FF and T2. The amplification condition was: 20 mM Tris-HCl (pH 8.8), 10 mM KCl, 10 mM (NH4)2SO4, 2 mM MgSO4, 0.1% Triton X-100, 50 μM dATP, 50 μM dTTP, 50 μM dCTP, 50 μM dGTP, 400 nM primer T1 and 400 nM primer 139FR (for fragment T1FR) or 400 nM primer T2 and 400 nM primer 139FF (for fragment FFT2), 1.5 U Pfu DNA polymerase (Promega, USA), ...
example 3
Site-Directed Mutagenesis of Arg182 of Glucose Isomerase
[0027]The site directed mutagenesis was done as described by Ho et al., Gene 77:51-59, 1989 and White et al., PCR protocol: current methods and applications. Totowa, N.J.: Humana Press 1993.
[0028]Using pGEMT-TS (Example 1) as template, the Arg (R) at position 182 of the wild-type glucose isomerase was mutated to Ala (A) to generate glucose isomerase mutant MGI-R182A by PCR amplification with site-directed primers 182AF and 182AR (Table 1) and universal primers T1 and T2 (Example 1).
[0029]Fragment T1AR was amplified using primer pair T1 and 182AR. Fragment AFT2 was amplified using primer pair 182AF and T2. The amplification condition was: 20 mM Tris-HCl (pH 8.8), 10 mM KCl, 10 mM (NH4)2SO4, 2 mM MgSO4, 0.1% Triton X-100, 50 μM dATP, 50 μM dTTP, 50 μM dCTP, 50 μM dGTP, 400 nM primer T1 and 400 nM primer 182AR or 400 nM primer T2 and 400 nM primer 182AF, 1.5 U Pfu DNA polymerase, 20 ng pGEMT-TS, and the total volume was adjusted t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com