Artificial tears and therapeutic uses
a technology of artificial tears and therapeutic uses, applied in the field of artificial tears, can solve the problems of eye surface, increased discomfort and sensitivity to bright light, and affects both eyes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Manufacturing Procedure for Emulsion Compositions of this Invention
[0033]As disclosed below, the emulsion compositions of this invention may be manufactured with and without tonicity components, such as glycerin, erythritol, carnitine, etc.
[0034]Batches were manufactured on a weight basis from pre-weighed compendial raw materials in five parts:
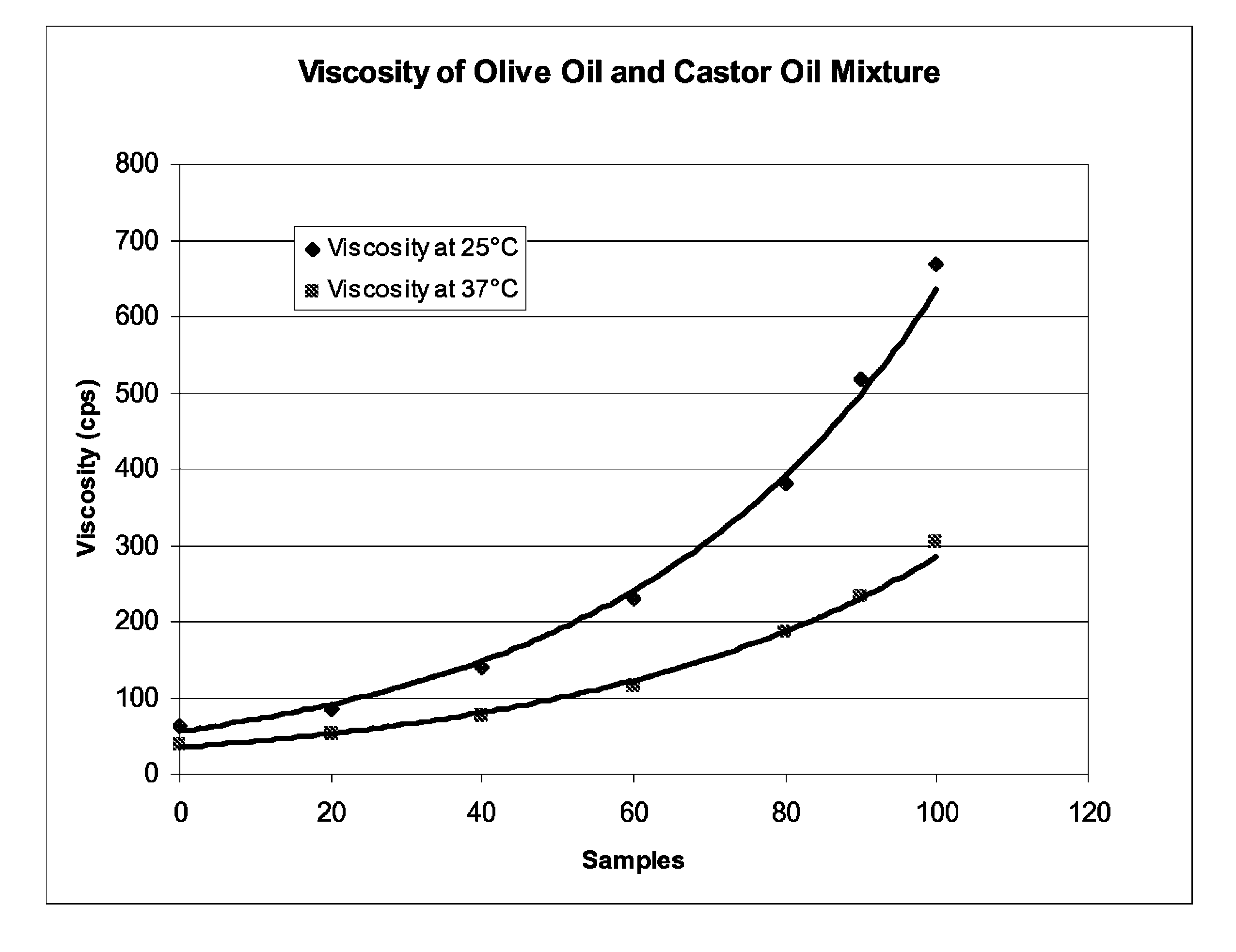
[0035]Part 1: an oil phase containing a mixture of castor oil and olive oil (non-sterile).
[0036]Part 2: an aqueous phase containing purified water, polysorbate 80, and glycerin. Part 2 is sterile filtered using a sterile Nalgene filtration unit with a 0.2 μm PES membrane.
[0037]Part 3: an aqueous polymer dispersion containing purified water and Pemulen® TR-2
(5× Stock solution, 0.5%). Part 3 is sterilized by autoclaving at 121° C. for 15 minutes at 15 psig.
[0038]Part 4: an aqueous polymer dispersion containing purified water and sodium carboxymethylcellulose. (5× Stock solution, 2.5%). Part 4 is sterilized by autoclaving at 121° C. for 15 minute...
example 2
Testing of Oil Mixtures for Ability to Spread on Ocular Surface Drop Dispersion Procedure
[0049]1. “Model Tear Solution”, described in Table 2, below, is added to of a small cell culture dish (25 mm×10 mm style) in an amount sufficient to cover the bottom thereof. Amount should be ˜2.5 mL);[0050]2. One drop of oil mixture (10 to 50 μl drop size) is added to said dish and an observation of how readily the drop disperses on the surface of the tear solution is made for not less then 60 seconds;[0051]3. The drop dispersed on the tear solution surface is mixed with a spatula and how readily the drop disperses in the tear solution is observed; and,[0052]4. The mixed oil and tear solution observe for several minutes / hours to see if oil coalesces.
[0053]
TABLE 2Composition of Model Tear SolutionIngredientConc. (% w / v)Sodium Chloride0.90Calcium Chloride, Dihydrate0.015Sodium Phosphate Dibasic, Heptahydrate0.028Lysozyme, egg white0.19Albumin, bovine0.020Gamma-Globulin, human0.010Mucin, bovine su...
example 3
Summary of 1-Day Ocular Tolerability Study of an Emulsion of Example 2 in Rabbits
[0058]A 1-day ocular tolerability study of an emulsion comprising 0.25%, by weight, of a 70 / 30 castor oil / olive oil mixture, manufactured according to Example 1, was conducted in rabbits. The oil mixture was utilized as a vehicle for the tonicity components carnitine, glycerine and erythritol. Female New Zealand White rabbits (5 rabbits / group) were given one drop (˜40 μL each drop) of said emulsion of or a marketed, comparator eye drop, comprising 1.25%, by weight, castor oil, emulsified in an aqueous phase, by topical ocular instillation in the left eye (OS), 6 times daily (approximately 1 hr intervals) for one day. The contralateral right eye (OD) served as a control without eye drop instillation. The following parameters were evaluated: viability, clinical observations, ocular discomfort, gross ocular observations (irritation), and ophthalmic examination (slit lamp biomicroscopy, pupillary reflex). O...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com