Nucleic acids encoding an endometrial bleeding associated factor (EBAF)
a gene and endometrial technology, applied in the field of human bodily conditions diagnosis, can solve the problems of false positive, increased blood loss in the gastro-intestinal tract, false positive, etc., and achieve the effects of accurate, reliable method, accurate, reliable method, and increased expression of the ebaf gen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Detection of Mucinous Adenocarcinomas of the Colon
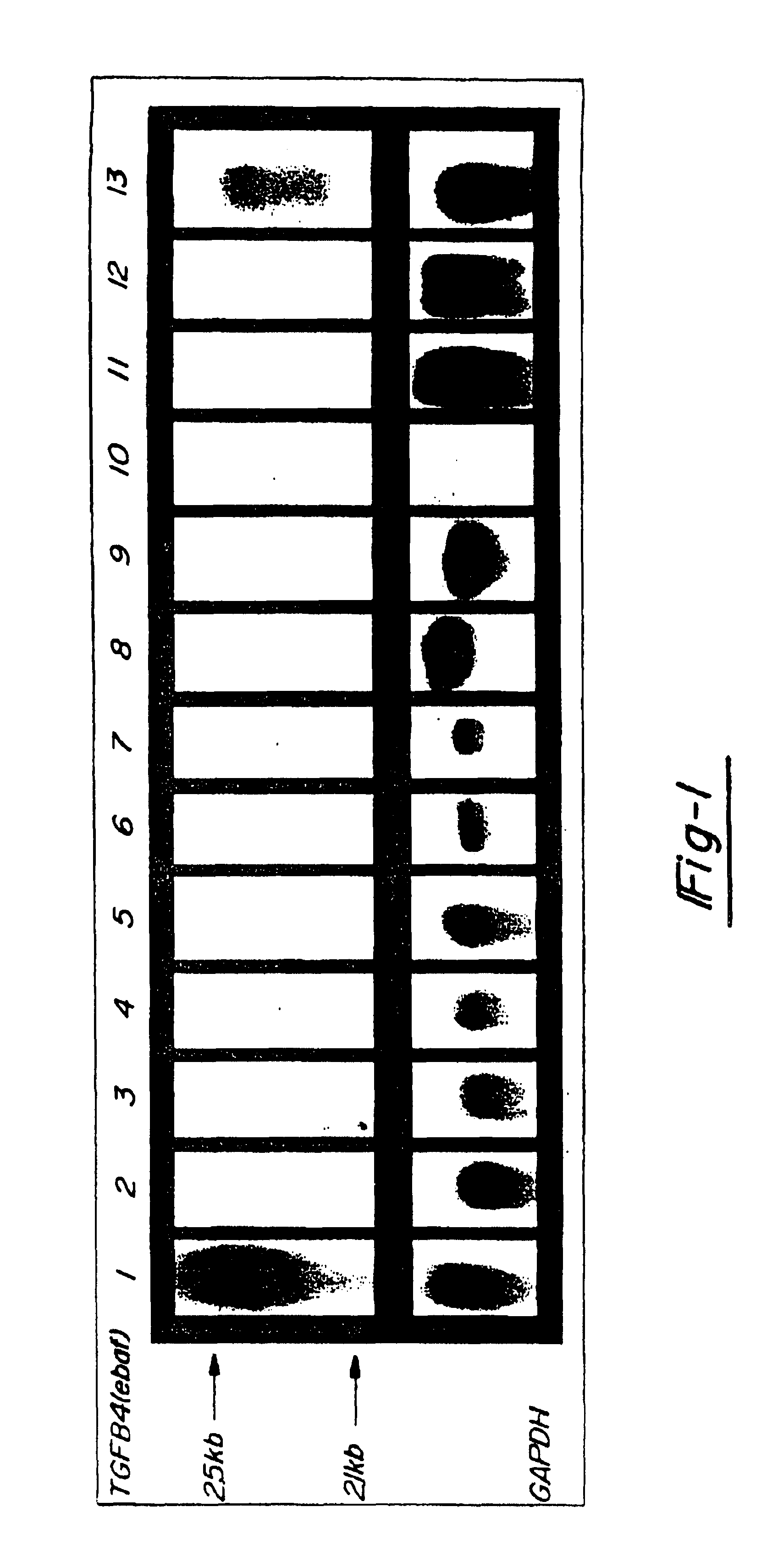
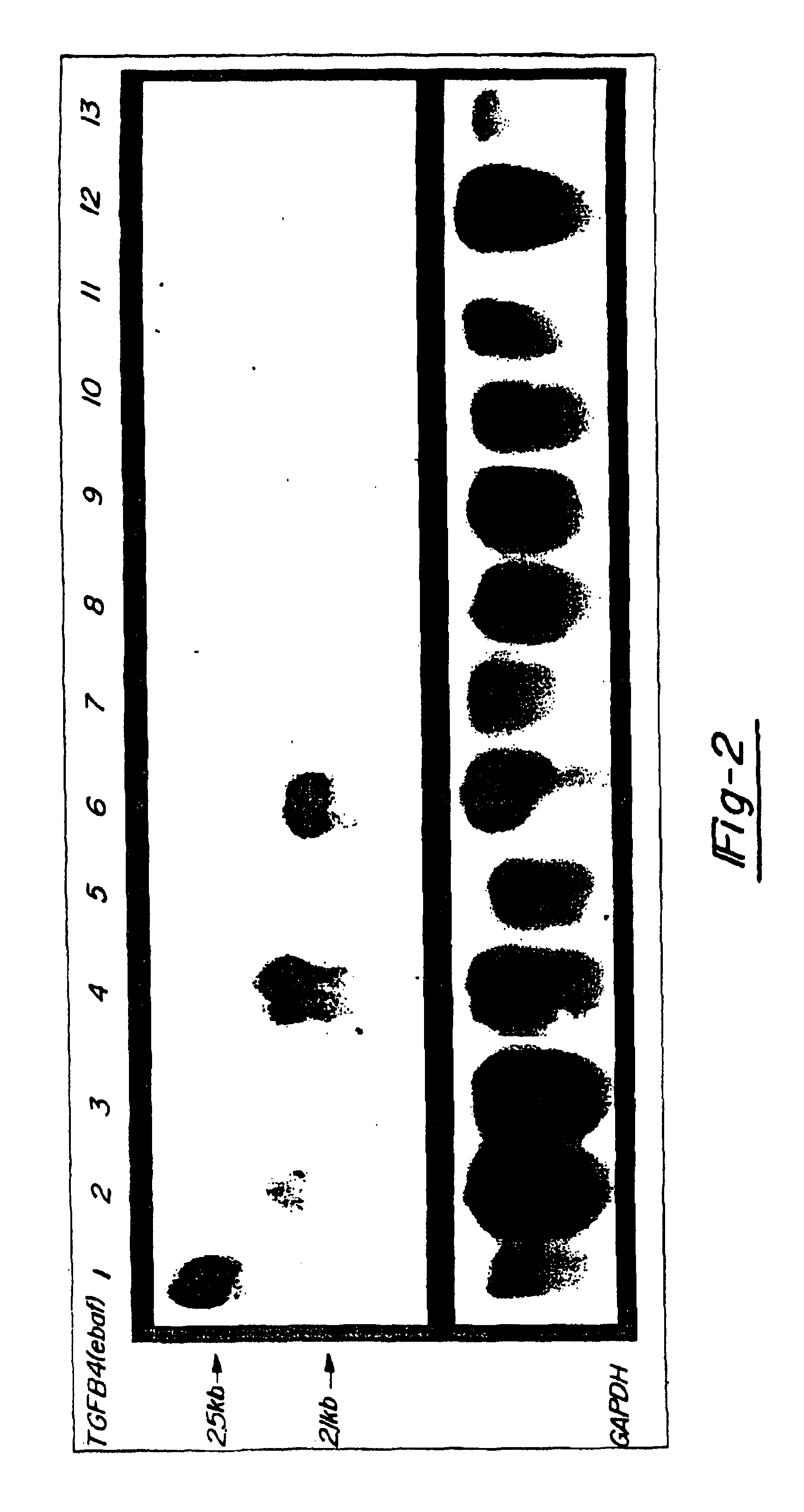
[0042]In FIG. 2, 20 mg total RNA from a normal late secretory endometrium which served as the positive control (lane 1) as well as mucinous adenocarcinomas of colon (lanes 2,4,6), non-mucinous adenocarcinomas of colon (lanes 8, 10, and 12) and adjacent normal colon (lanes 3, 5, 7, 9, 11 and 13) was subjected to the Northern blot analysis using the entire placental-derived ebaf cDNA as the probe (upper panel). Here, the expression of a 2.1 kb ebaf mRNA was detected in seven of the eleven cases of adenocarcinomas of the colon. The histological evaluation of the positive cases revealed them to have a mucinous differentiation.
example 2
Detection of Adenocarcinomas of the Testis
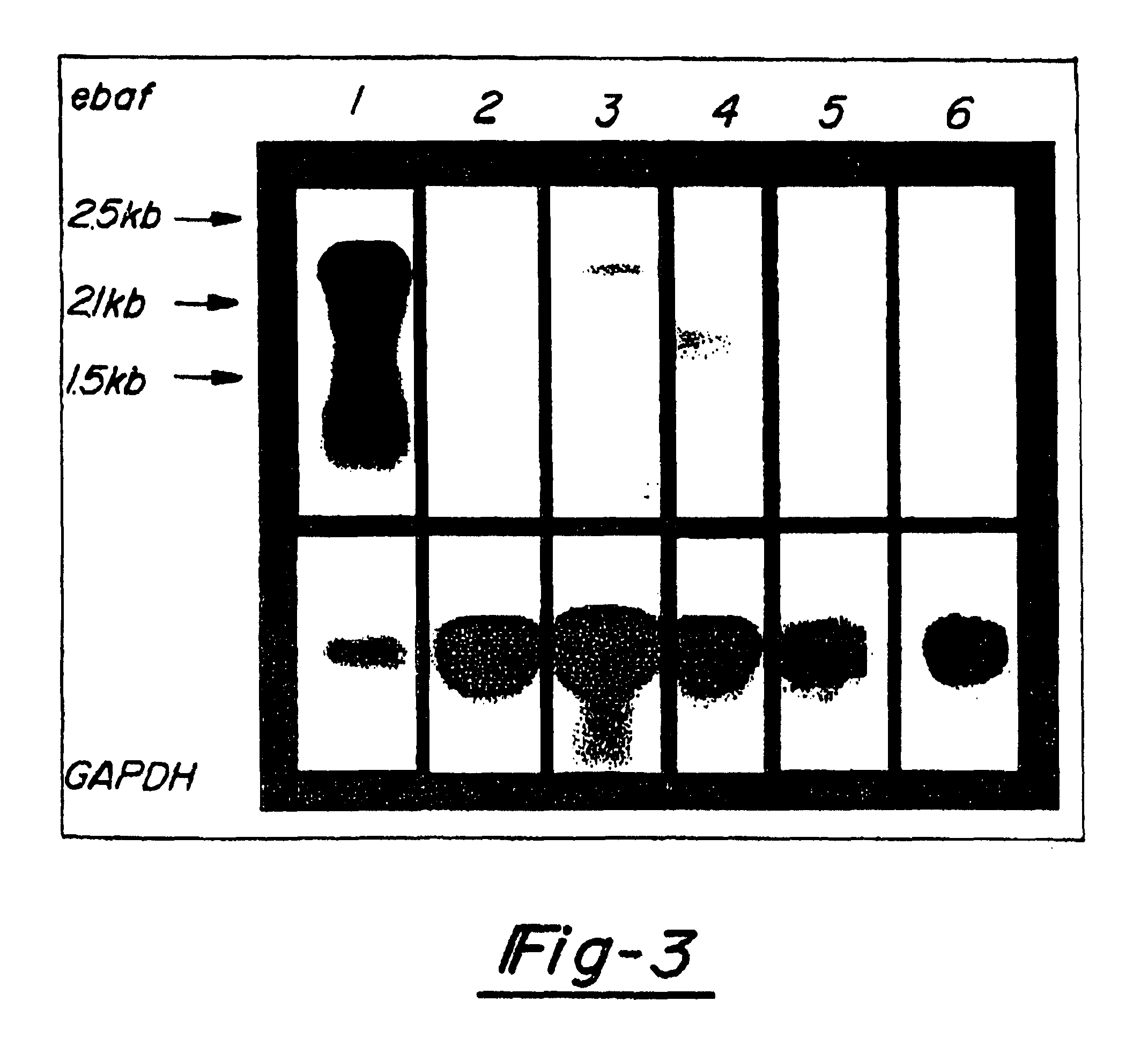
[0043]FIG. 3 is a Northern blot displaying the results of an examination of five cases of testicular cancer for the expression of ebaf gene. 20 mg of total RNA from a normal menstrual endometrium which served as the positive control (lane 1) and each tumor tissue (lane 2: teratoma-embryonal cell carcinoma, lane 3: mixed germ cell tumor containing embryonal carcinoma, lanes 4-6; seminoma) were subjected to the Northern blot analysis using the entire placental-derived ebaf cDNA as the probe (upper panel). The blot was exposed for long duration to detect ebaf mRNA in the neoplastic tissues. This resulted in the overexposure of the ebaf mRNAs detected in the endometrium.
[0044]The results shown in FIG. 3 indicate that a 2.5 kb ebaf mRNA is detected in the tumors containing embryonal carcinoma. The 2.1 kb mRNA is also detected in two out of three cases of seminoma. The integrity of RNA and equal loading was verified by staining the 18S and 28S rib...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com