Method for determining contents of six components of mango cough-relieving tablet by HPLC QAMS (quantitative analysis of multi-components by single-marker) method
A mango and content technology, which is applied in the field of HPLC method for the determination of the content of 6 components in Mango Zhike Tablets by one measurement and multiple evaluations, can solve the problems of high detection cost, complex components and high price, and achieves accurate and reliable method, good repeatability and repeatability. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
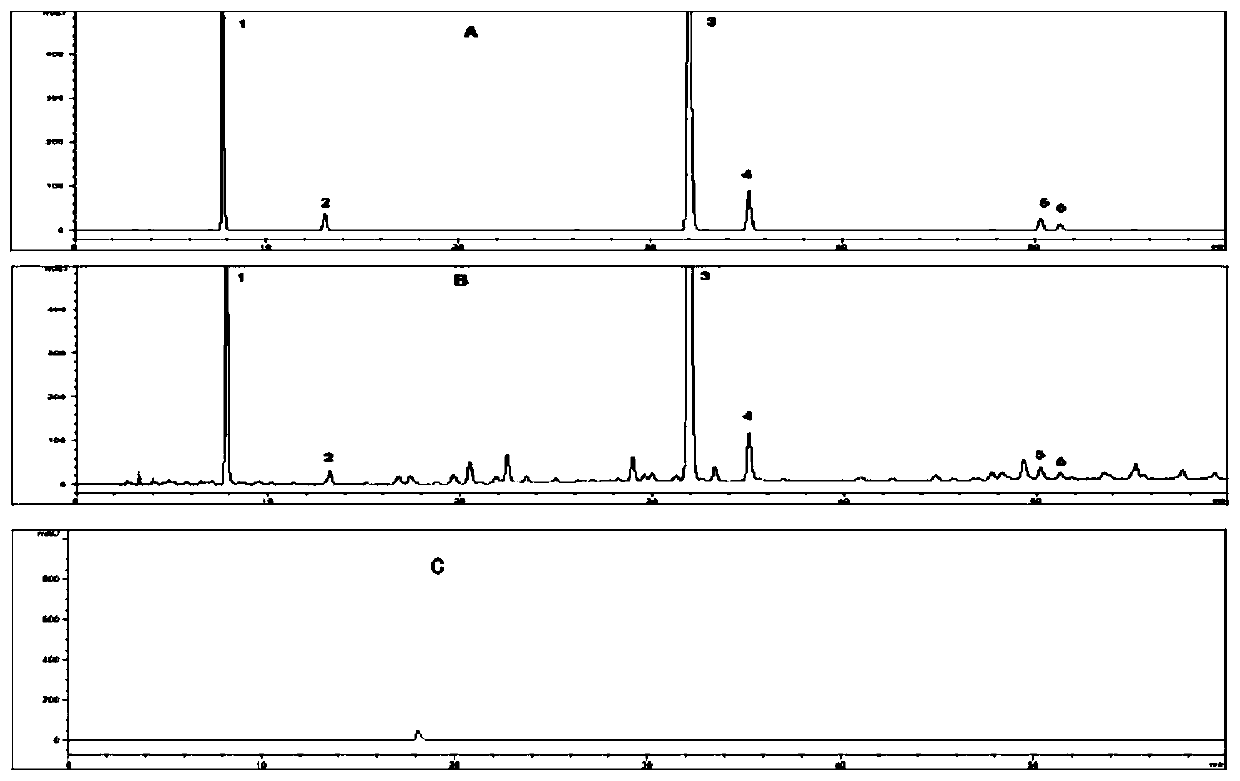
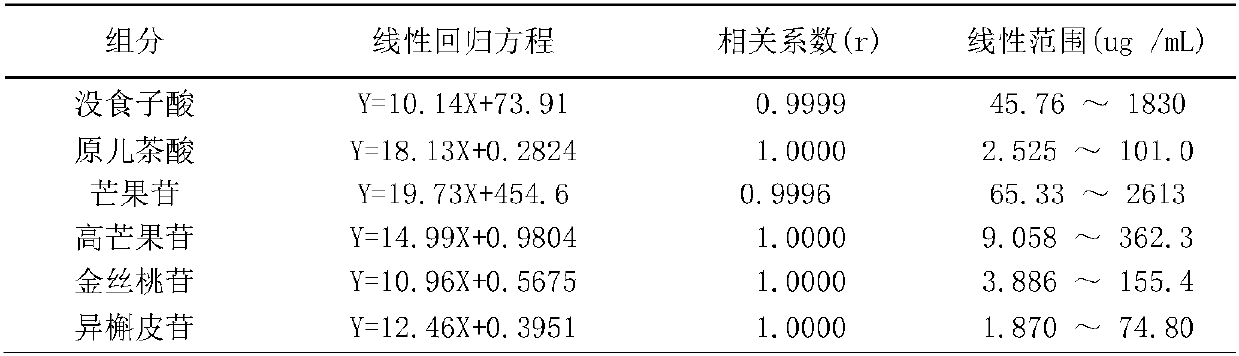
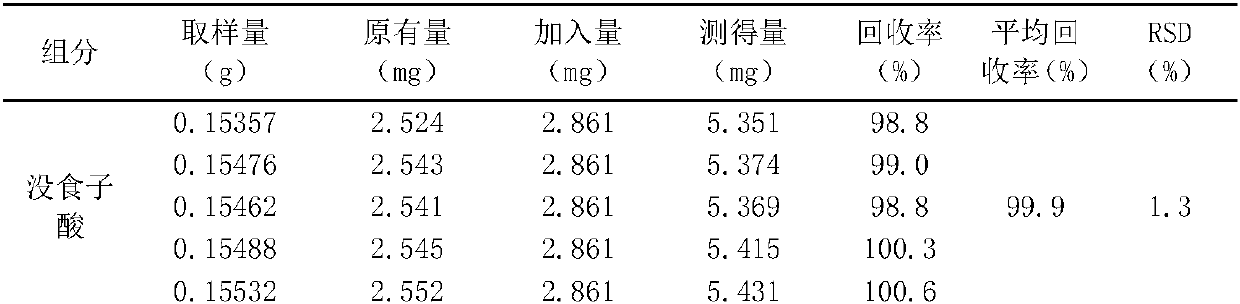
Image
Examples
Embodiment Construction
[0021] 1. Experimental materials
[0022] 1.1 Experimental reagents, drugs and medicinal materials
[0023] The 11 batches of mango cough tablets were all provided by Guangxi University of Traditional Chinese Medicine Pharmaceutical Factory (batch numbers: 20161201, 20161202, 20170304, 20170401, 20170501, 20170901, 20180601, 20180602, 20180701, 20180702, 20181001). Gallic acid (batch number: 110831-201605, with a mass fraction of 90.8%) and protocatechuic acid (batch number: 110809-201205, with a mass fraction of 99.9%) were purchased from China Institute for Food and Drug Control; high mangiferin (batch number ST216701, purity ≥ 98%), hyperoside (batch number ST008801, purity ≥ 98%), isoquercitrin (batch number: ST114401, purity ≥ 98%) were all purchased from Shanghai Standart Standard Technology Co., Ltd.; mangiferin (batch number wkq18050201, HPLC≥98%) was purchased from Sichuan Weikeqi Biotechnology Co., Ltd. Mango leaf control medicinal material (batch number: 121507-20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com