Method for producing specific crystalline modifications of polymorphous substances
A crystal, a specific technology, applied in the fields of botanical equipment and methods, separation methods, crystallization bed crystallization, etc., can solve the problems of high processing cost and time-consuming development.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
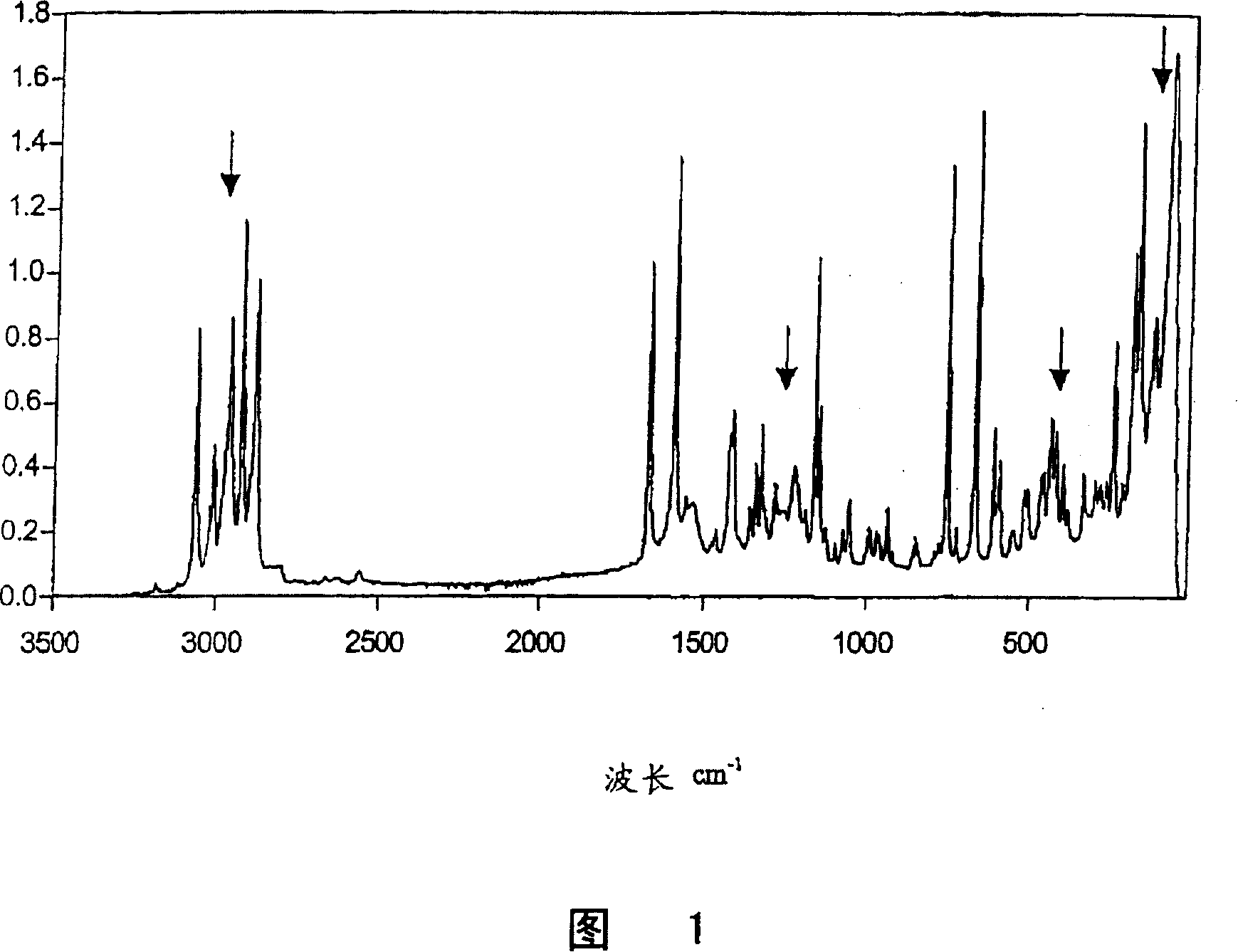
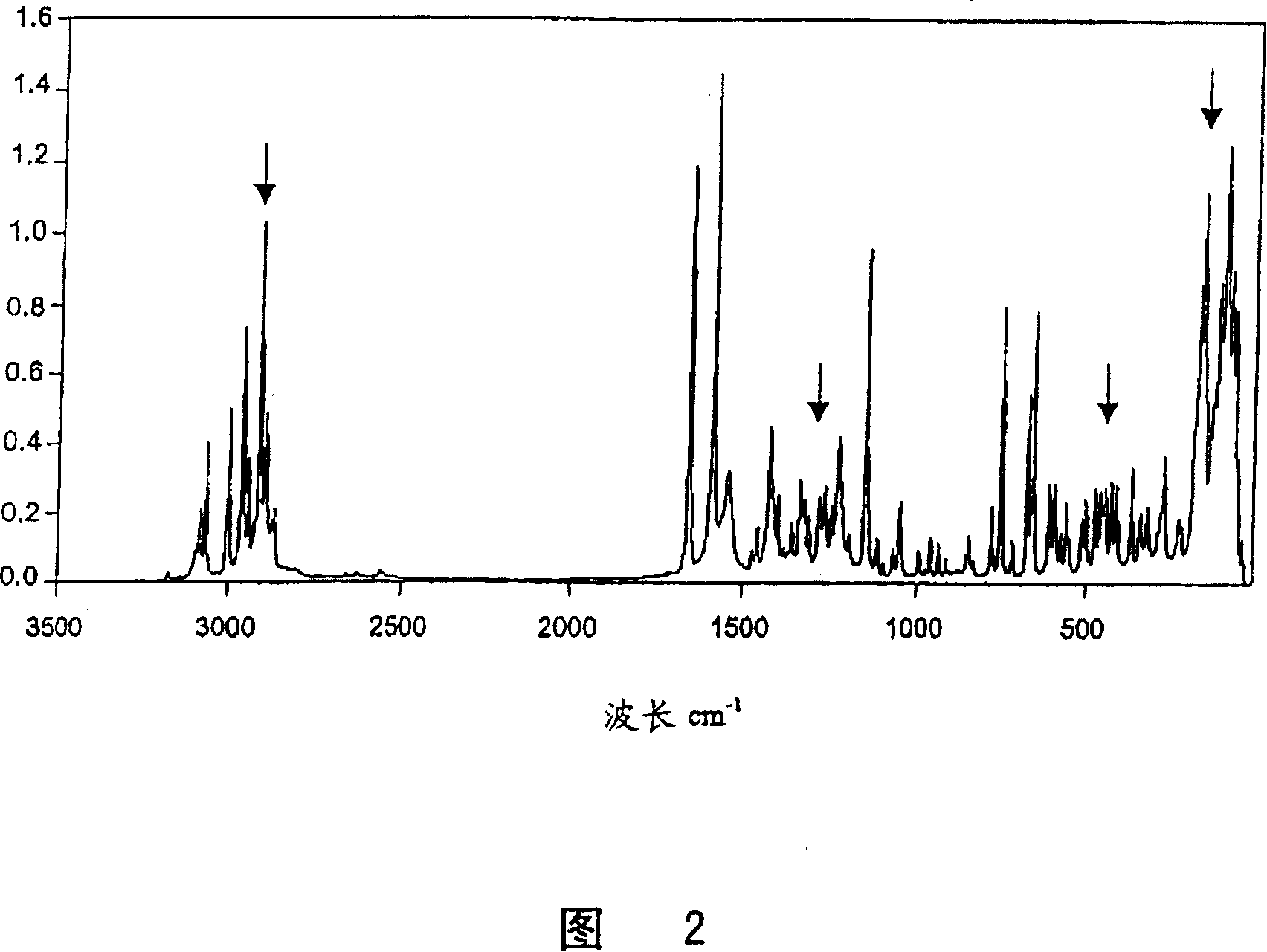
[0036] The stability of 2-(2-chloro-4-methylsulfonyl-benzoyl)cyclohexane-1,3-dione (sulcotrione) Preparation of a given crystal structure (modification II) by direct precipitation (additive: heterocyclic amine)
[0037] A toluene suspension of the triethylamine salt of 2-(2-chloro-4-methylsulfonyl-benzoyl)cyclohexane-1,3-dione, with the aid of the corresponding enol ester 3-oxo-cyclo Prepared by rearrangement of hex-1-enyl-(2-chloro-4-methanesulfonylbenzoate) (see EP-A2-186 117). The enol ester is here obtained by reacting 0.2 mol of 2-chloro-4-methanesulfonyl-benzoyl chloride with an equimolar amount of cyclohexane-1,3-dione in toluene in the presence of an excess of triethylamine as base synthesized by coupling. This toluene suspension was contacted with 500 g of dilute sodium hydroxide (4% by weight) at 45° C. for 30 min in order to obtain, as product, the sodium salt dissolved in the aqueous phase and free of by-products. After phase separation, the aqueous sodium en...
example 2
[0041] The stability of 2-(2-chloro-4-methylsulfonyl-benzoyl)cyclohexane-1,3-dione (sulcotrione) Preparation of a given crystal structure (modification II) by direct precipitation (additive: ketone)
[0042] The procedure described in Example 1 was carried out in an analogous manner but with a ketone as an additive in order to obtain 2-(2-chloro-4-methanesulfonyl-benzoyl)cyclohexane-1 directly by precipitation crystallization from the corresponding aqueous solution of sodium enolate, Stable crystal structure of 3-diketone (modification II). The crystal structure thus obtained was determined to be characterized by the characteristic Raman spectrum given in Example 1. The results of the ketones used, amounts and methods are shown in Table I.
[0043] Table I
[0044] example
example 3
[0046] The stability of 2-(2-chloro-4-methylsulfonyl-benzoyl)cyclohexane-1,3-dione (sulcotrione) Preparation of a given crystal structure (modification II) by direct precipitation (additive: alcohol)
[0047] The procedure described in Example 1 was carried out in a similar manner but with various alcohols as additives in order to obtain 2-(2-chloro-4-methanesulfonyl-benzoyl)cyclohexane directly by precipitation crystallization of the corresponding aqueous sodium enolate solution- Stable crystal structure of a 1,3-diketone (modification II). The crystal structure thus obtained was determined to be characterized by the characteristic Raman spectrum given in Example 1. The results of the alcohols used, amounts and methods are given in Table II.
[0048] Table II
[0049] example
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com