High level production of P-hydroxybenzoic acid in green plants
A technology of p-hydroxybenzoic acid and hydroxybenzoic acid glucoside, which is applied in the field of producing p-hydroxybenzoic acid and can solve the problems of enzyme activity loss and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] PCR Cloning of Escherichia coli CPL
[0108] The E. coli ubiC gene was amplified from genomic DNA using two PCR primers while adding unique restriction sites to its flanking regions for subsequent ligation into a high copy number plasmid. This gene encodes chorismate pyruvate lyase, hereinafter referred to as CPL. The primers used for this purpose were based on the published DNA sequence of the E. coli ubic gene (GenBank accession number M96268) and consisted of the following nucleotides:
[0109] Primer 1-(SEQ ID NO: 1):
[0110] 5′-CTA CTC ATT Tca tat gTC ACA CCC CGC GTT AA - 3' Primer 2- (SEQ ID NO: 2):
[0111] 5′-CAT CTT ACT aga tct TTA GTA CAA CGG TGA CGC C - 3' underlined bases hybridize to the target gene, while lower case letters indicate restriction sites (NdeI or BglII) added to the ends of the PCR primers.
[0112] Amplification of the E. coli ubic gene was accomplished using primers 1 and 2 and genomic DNA from E. coli strain W3110 (Campbell et al....
Embodiment 2
[0115] Overexpression, Purification and Characterization of Recombinant Escherichia coli CPL
[0116] To generate sufficient CPL for enzyme characterization and antibody production, pET24a-CPL was introduced into E. coli BL21(DE3). This step was accomplished by electroporation using a BTX Transfector 100 (Biotechnologies and Experimental Research Inc.), according to the manufacturer's protocol. Growths were selected on LB medium containing kanamycin (50 μg / ml) and one colony was selected for further manipulation. To produce the recombinant protein, the strain containing the plasmid was grown in liquid culture in the above medium at 30°C in A 600nm At about 0.8, cells were induced with 0.15 mM IPTG. After a 4.5 hour induction period under the same growth conditions, cells were harvested by centrifugation and stored at -80°C until use. Subsequent steps were performed at 0-4°C.
[0117] Resuspend the frozen cell pellet in approximately 3 volumes of 0.1M Tris-HCl (pH 7.7), ...
Embodiment 3
[0122] A chloroplast-targeted form of CPL: construction of TP-CPL
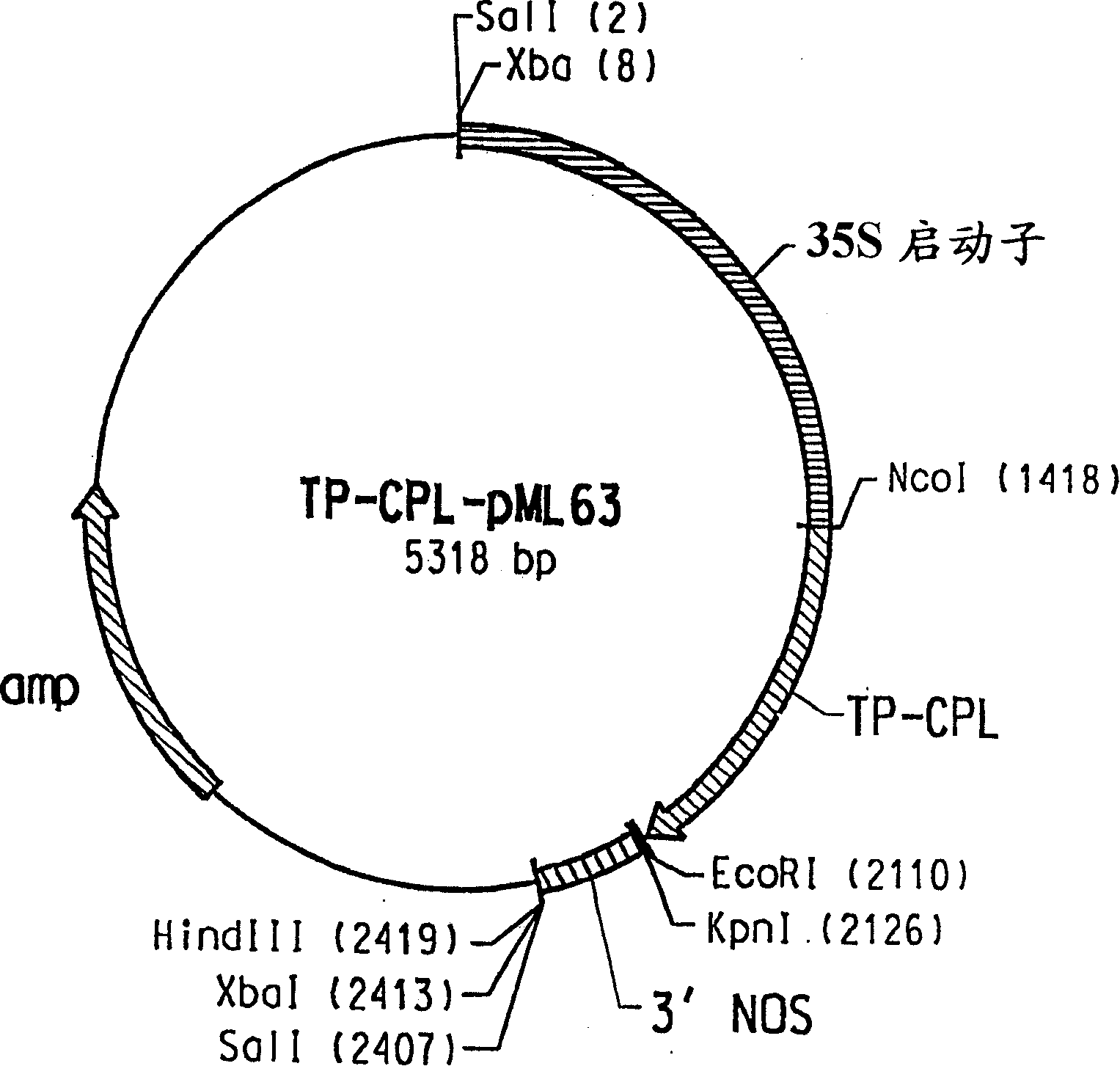
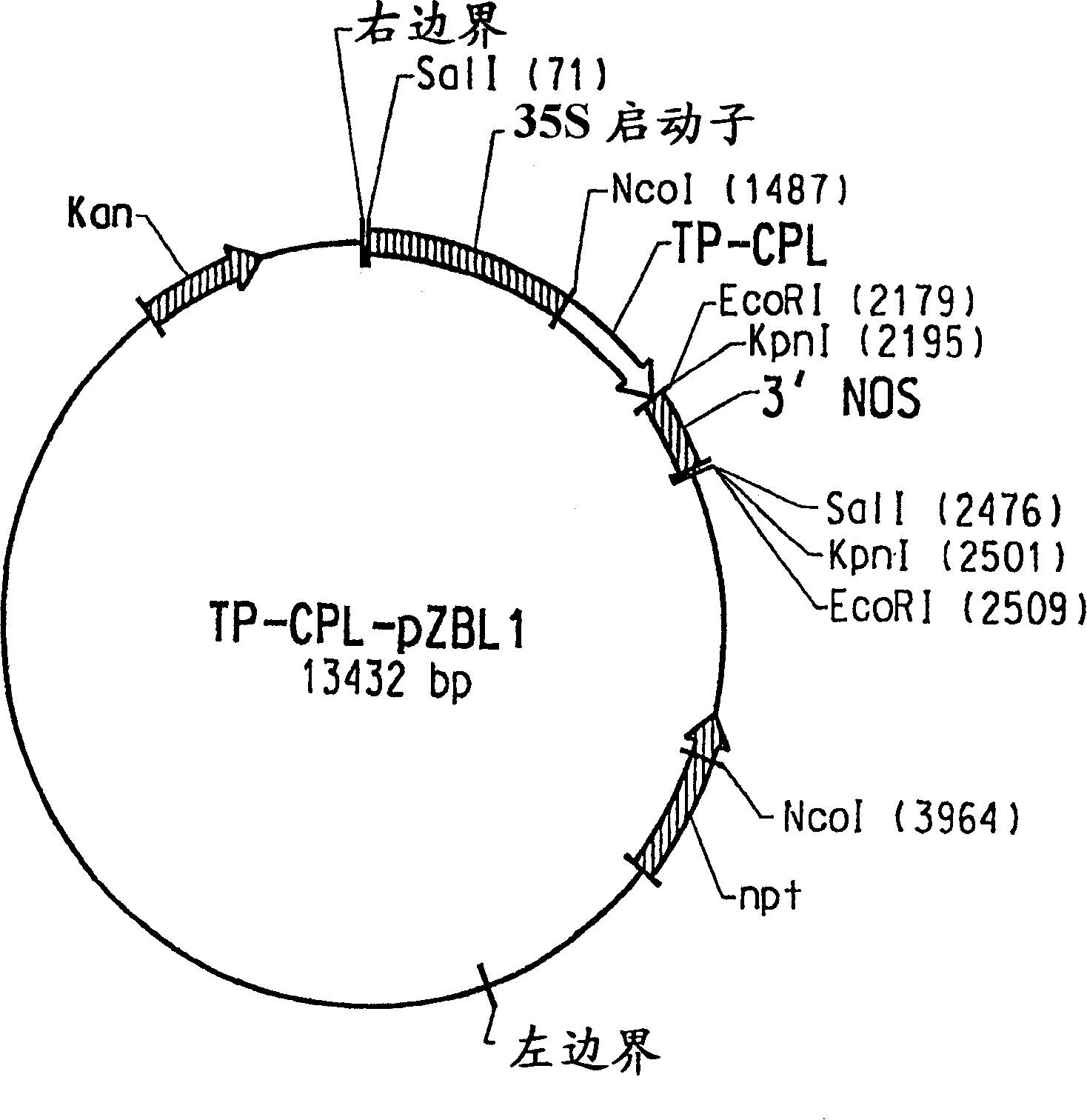
[0123] Chorismic acid is the physiological substrate of CPL, and it is an important branch point intermediate in the synthesis of various aromatic compounds including the amino acids phenylalanine and tyrosine. In plants, chorismate is produced in the shikimate pathway located in chloroplasts and other types of plastids (Siebert et al., Plant Physiol. 112:811-819 (1996)). Therefore, it is necessary to provide CPL with an N-terminal chloroplast targeting sequence capable of efficiently directing foreign proteins to the chloroplast, the site of chorismate production. This is accomplished by constructing a chimeric protein consisting of a chloroplast targeting sequence derived from the tomato Rubisco small subunit precursor protein fused to the initiator Met residue of CPL; the resulting fusion protein is hereinafter referred to as "TP -CPL". To generate DNA fragments corresponding to the Rubisco small subuni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com