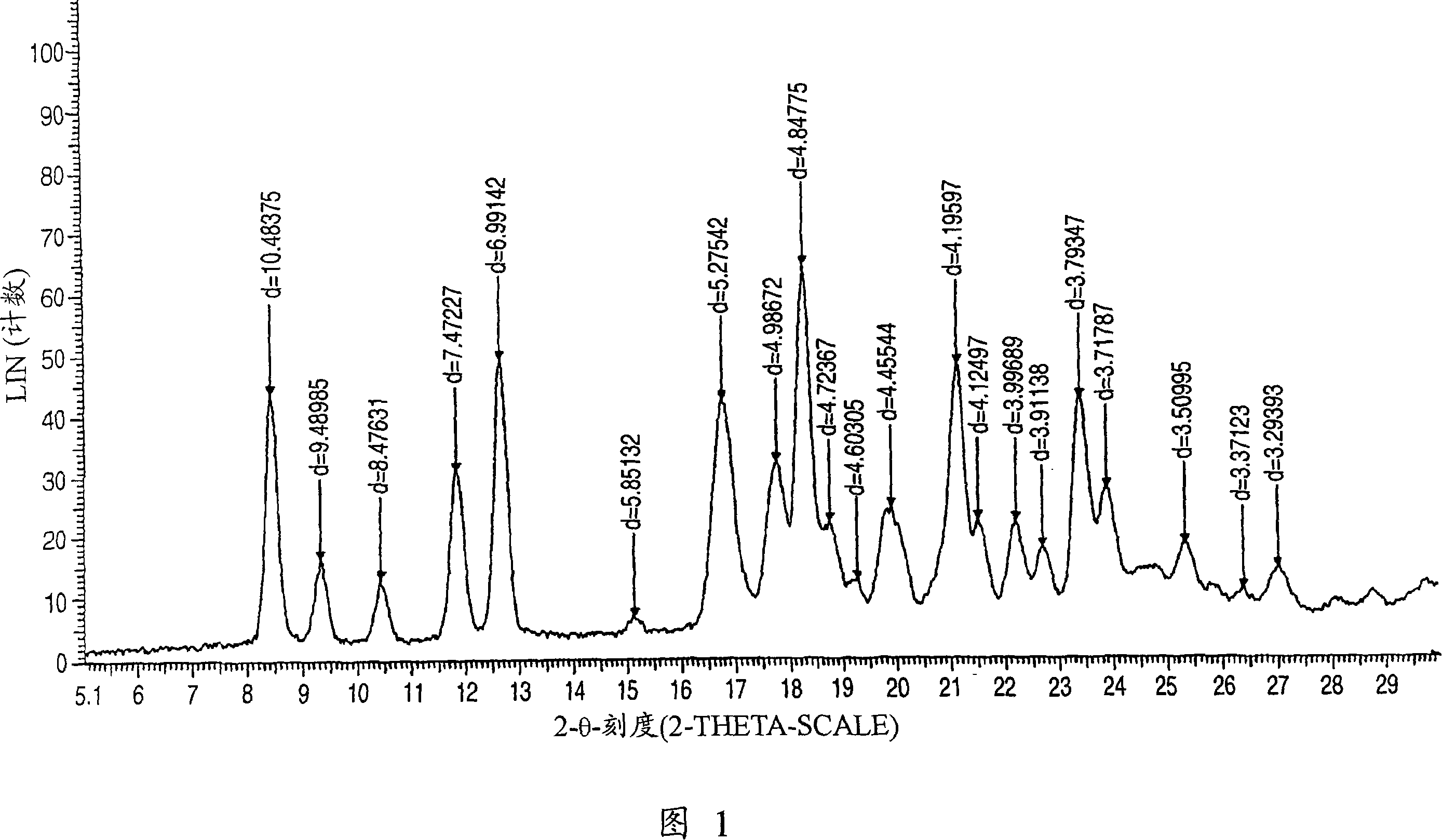
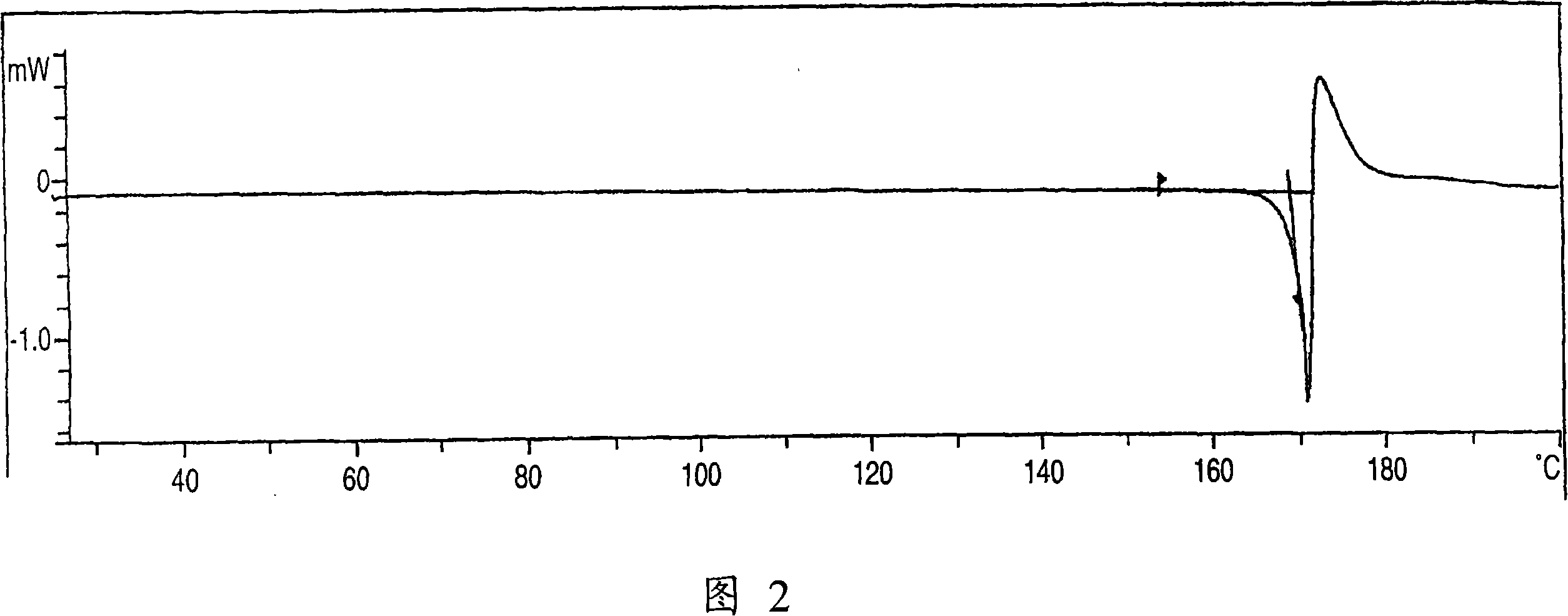
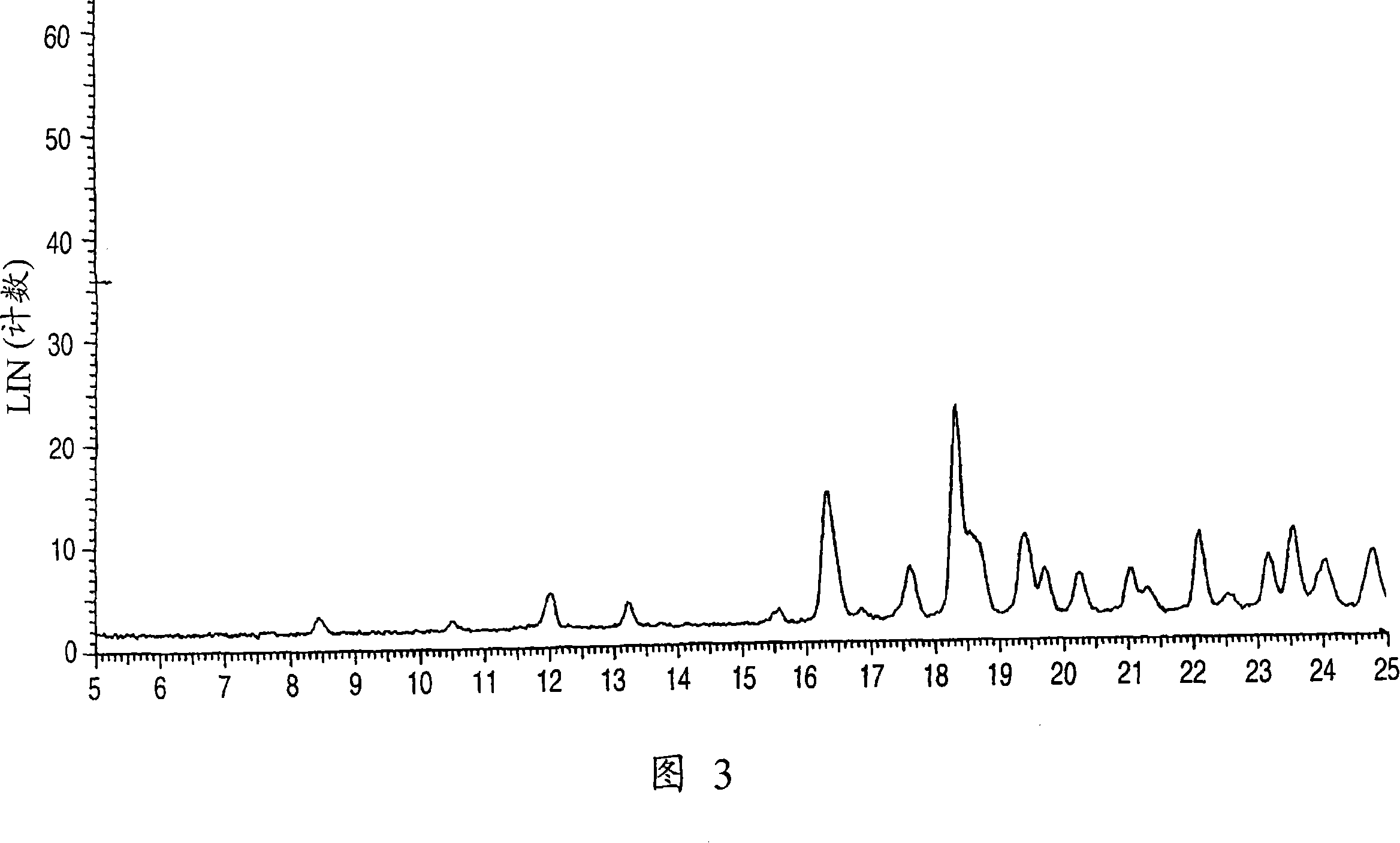
Aminocarboxylic acid derivative and medicinal use thereof
A technology of azetidine carboxylic acid and objects, applied in the field of aminocarboxylic acid derivatives and their medical applications, can solve the problem of no EDG-6 agonist or antagonist
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0445] Example 1: 6-(Benzyloxy)-3,4-dihydronaphthalene-1(2H)-one
[0446] To a solution of 6-hydroxy-3,4-dihydronaphthalen-1(2H)-one (24.3 g) in acetone (160 mL) was added benzyl bromide (29.4 mL) and potassium carbonate (31.1 g) at room temperature, followed by Stir at 40°C for 3.5 hours. After filtering off insolubles and concentrating the filtrate, the residue was washed with a mixed solvent of tert-butylmethyl ether-hexane (1:4) to obtain the title compound (34.5 g) having the following physical properties.
[0447] TLC: Rf0.38 (hexane: ethyl acetate = 3: 1)
Embodiment 2
[0448] Example 2: 7-(Benzyloxy)-4-methyl-1,2-dihydronaphthalene
[0449] To a solution of the compound prepared in Example 1 (34.5 g) in tetrahydrofuran (300 mL) was added methylmagnesium bromide (3 mol / L ether solution, 55 mL) at 0°C, followed by stirring at room temperature for 1 hour. The reaction mixture was cooled to 0°C and poured into ice-saturated aqueous ammonium chloride. After adding 2 mol / L hydrochloric acid, the mixture was stirred at room temperature for 3 hours. Thereafter, the product was extracted with ethyl acetate, and the organic layer was washed successively with water and saturated brine, dried, and then concentrated. The resulting residue was purified by silica gel column chromatography (hexane:ethyl acetate=10:1) to obtain the title compound (24.8 g) having the following physical properties.
[0450] TLC: Rf0.57 (hexane: ethyl acetate = 15:1)
Embodiment 3
[0451] Example 3: 6-(Benzyloxy)-1-methyl-3,4-dihydronaphthalene-2-carbaldehyde
[0452] N,N-Dimethylformamide (60 mL) was added dropwise to phosphorus oxychloride (26.7 g) at 0°C, followed by stirring for 20 minutes. Thereafter, a dichloromethane (60 mL) solution of the compound (24.8 g) prepared in Example 2 was slowly dropped thereinto, followed by stirring at room temperature for 90 minutes. The reaction liquid was cooled to 0° C., then poured into ice and left for a period of time. Next, the product was extracted with a mixed solvent of hexane-ethyl acetate (1:2). The organic layer was washed successively with water and brine, dried and concentrated. The obtained solid was washed with tert-butyl methyl ether to obtain the title compound (19.9 g) having the following physical properties.
[0453] TLC: Rf0.50 (hexane: ethyl acetate = 3: 1)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap