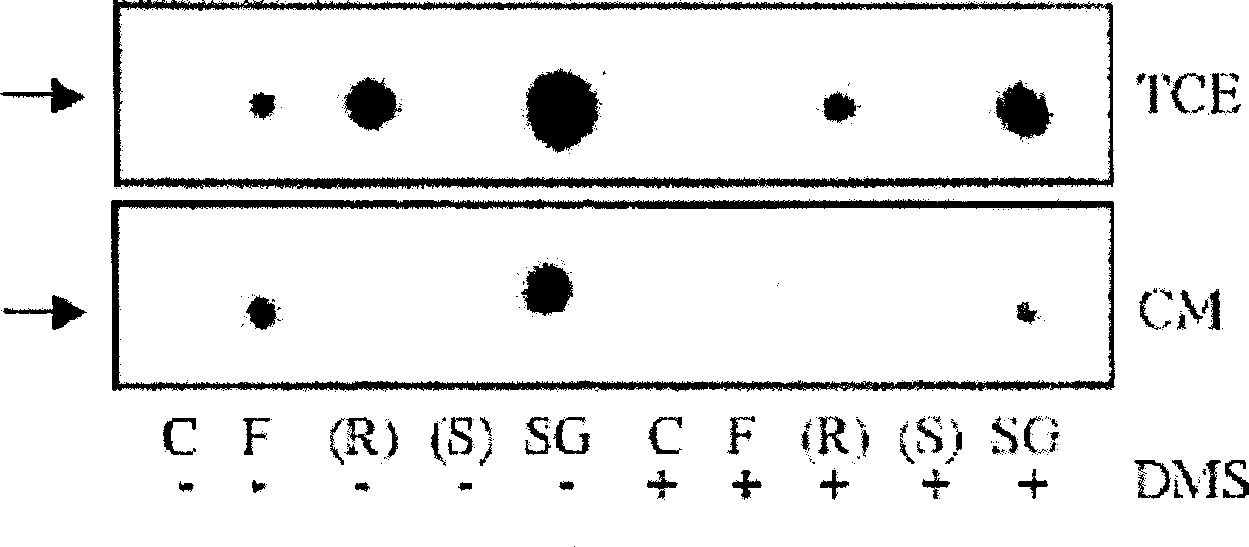Methods of inhibiting vascular permeability and apoptosis
A vascular and pharmaceutical technology, applied in chemical forms in the preparation of therapeutic and pharmaceutical applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1: Phosphorylation of FTY720 by Sphingosine Kinase in Vascular Endothelial Cells
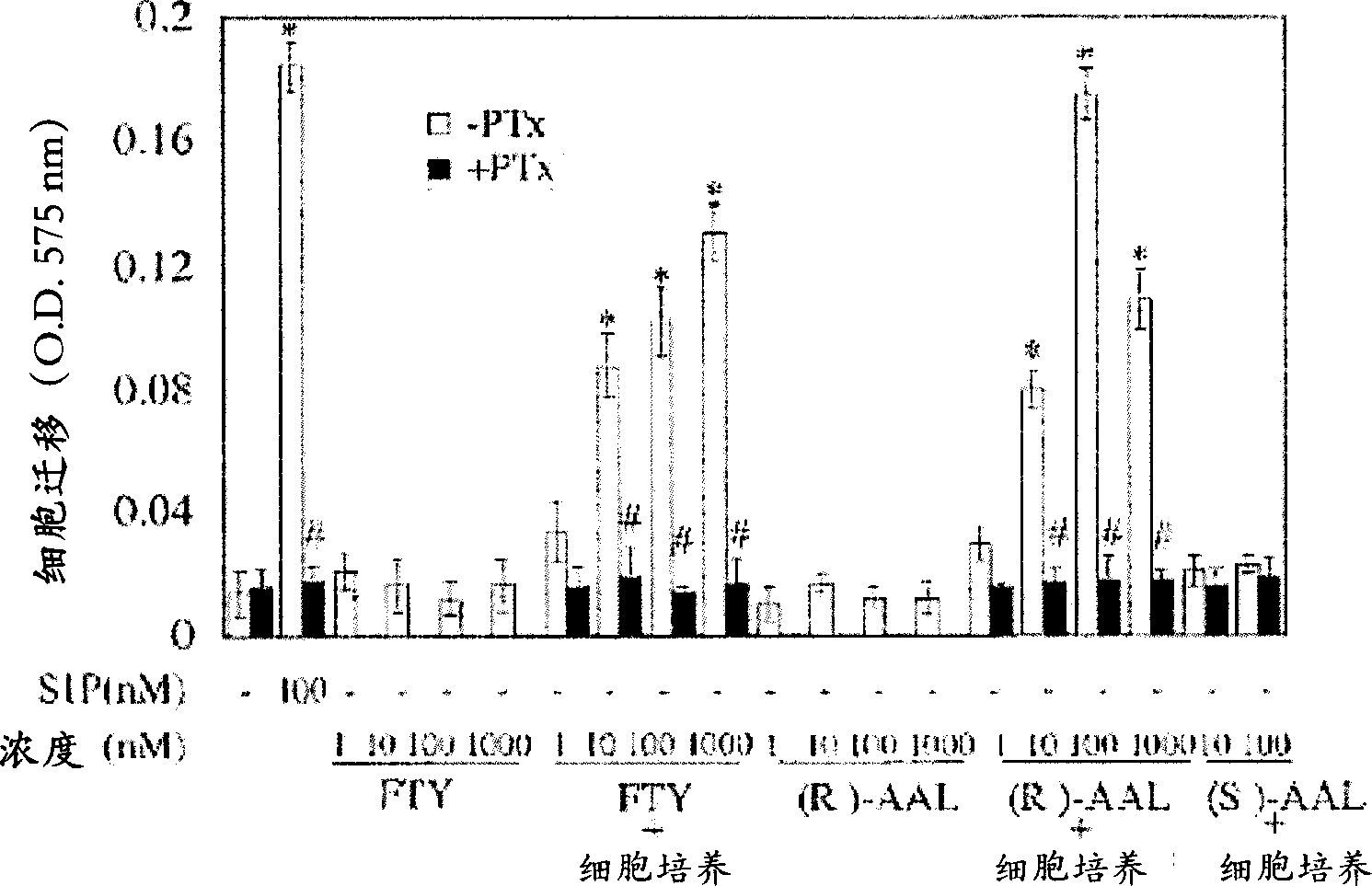
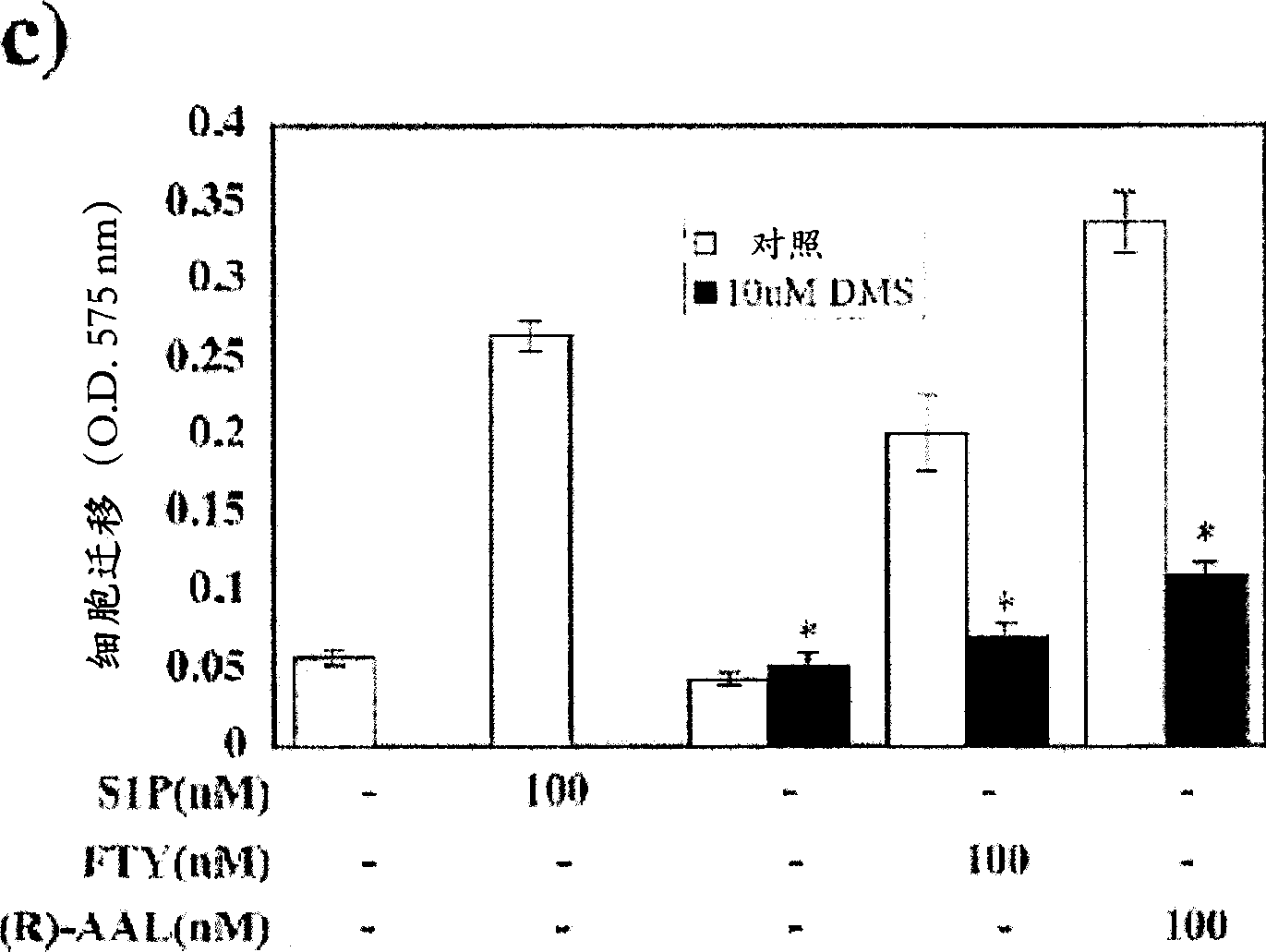
[0078] Since S1P is a potent inducer of endothelial cell chemotaxis, the effect of FTY720 and its analogues on endothelial cell migration was tested. HUVEC migration was analyzed using a 96-well chemotaxis microchamber (Neuro Probe Ltd.) (Paik, J.H. et al., J Biol Chem 276: 11830-11837 (2001)). Briefly, polycarbonate filters with a pore size of 8 microns ([mu]m) were coated with 5 micrograms per milliliter ([mu]g / ml) of fibronectin. S1P, FTY720, AAL, FTY720-P or (R)-AFD were diluted to an appropriate concentration with 0.1% ffa-BSA. Add 85 μl of solution or conditioned medium from HUVECs to the lower chamber. Approximately 5 x 10 4 cells were placed in the upper culture chamber. at 5% CO 2 Allow cells to migrate for 4 hr at 37 °C in a humid culture chamber. After the incubation period, the filters were removed, the cells were stained with 0.1% crystal violet in a 96-well pla...
Embodiment 2
[0084] Example 2: Confirmation of phosphorylation of FTY720 and R-AAL
[0085] To confirm that FTY720 and (R)-AAL can be phosphorylated by endothelial cells, in vitro kinase assays were performed using HUVEC whole cell extracts and conditioned medium. Cells were washed 3 times with M199 normal medium and incubated with 0.1% ffa-BAS for the indicated times. After incubation, conditioned medium was removed, centrifuged at 1,000 xg for 10 minutes and used for kinase activity assays. With 400μl 25mM HEPES pH7.5, 5mM MgCl 2 , 1X Protease Inhibitor Cocktail Scrape cells at 4°C and disrupt with brief sonication. The cell homogenate was centrifuged at 20,000 xg for 10 minutes at 4°C. In kinase assays, 300 μl of conditioned medium from HUVECs (derived from 5 × 10 5 cells were incubated for 3 hr) or 10 μg whole cell extract as a source of kinase activity. The reaction contained 20 μM sphingosine or 100 μM FTY720 or AAL, [γ- 32 P]ATP (10μCi), 5mM MgCl 2 , 15 mM NaF, 0.5 mM 4-deoxy...
Embodiment 3
[0087] Example 3: Determination of the role of SK2 in phosphorylation of FTY720 and (R)-AAL
[0088] To determine the role of SK2 in the phosphorylation of FTY720 and (R)-AAL, HEK293T cells were transiently transfected with pcDNA3.1 / Neo expression vector containing SK1 or SK2 cDNA, and FTY720, (R)-AAL and (S)- Phosphorylation of AAL. Figure 4 Shown are TLC-autoradiograms of in vitro kinase assays performed with extracts from control vector 293T cells (293T), SK1-overexpressing 293T cells (SK1-293T), and SK2-overexpressing 293T cells (SK2-293T) . Control vehicle = (C), 100 μM FTY720 = (F), 100 μM (R)-AAL = (R), 100 μM (S)-AAL = (S) or 20 μM sphingosine = (SG) were used as substrates. Contains 50 μM DMS when marked (+). Representative experiments for N=3-5 are shown. The bottom image shows SK1-293T (white bars) and SK2-293T (black bars) vs. The specific activity induction fold of vector transfection 293T. Arrows indicate phosphorylated compounds.
[0089] Moderate amount...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com